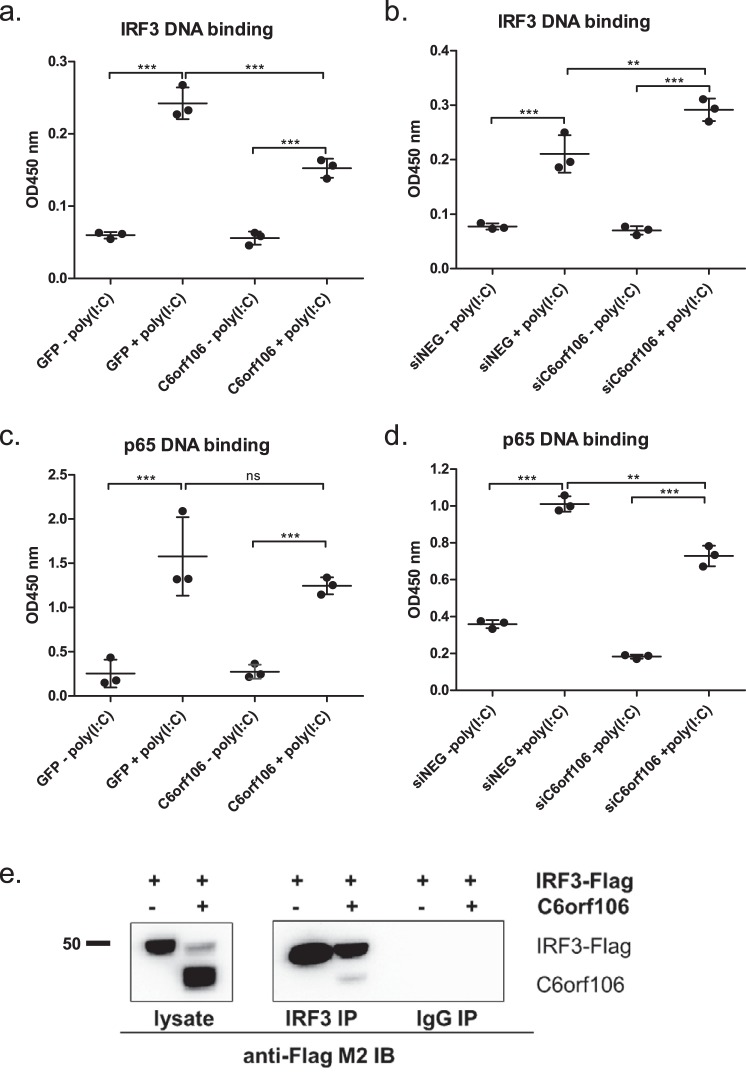
Figure 6.
C6orf106 interacts with IRF3 and inhibits binding to its DNA consensus sequence. HeLa cells were transfected with cDNAs and stimulated with poly(I:C), and the nuclear proteins were extracted using a hypotonic lysis method. Nuclear proteins (10 μg) were analyzed for IRF3–DNA (a) or p65–DNA (c) binding. Alternatively, HeLa cells depleted of C6orf106 were stimulated with poly(I:C), and nuclear proteins were analyzed for IRF3–DNA (b) or p65–DNA (d) binding. e, HEK293T cells transfected with IRF3 alone or in combination with C6orf106 were stimulated with poly(I:C), lysed, and subjected to indirect immunoprecipitation with an anti-IRF3 antibody. Immunoprecipitated (IP) samples and input controls were probed with anti-FLAG antibody for Western blotting. An IgG isotype was used as a negative control for the immunoprecipitation experiment. Error bars indicate ±1 S.D. of triplicate experiments; asterisks indicate significant differences as determined by two-way ANOVA with Bonferroni post-test (***, p < 0.001; **, p < 0.01; ns, not significant).