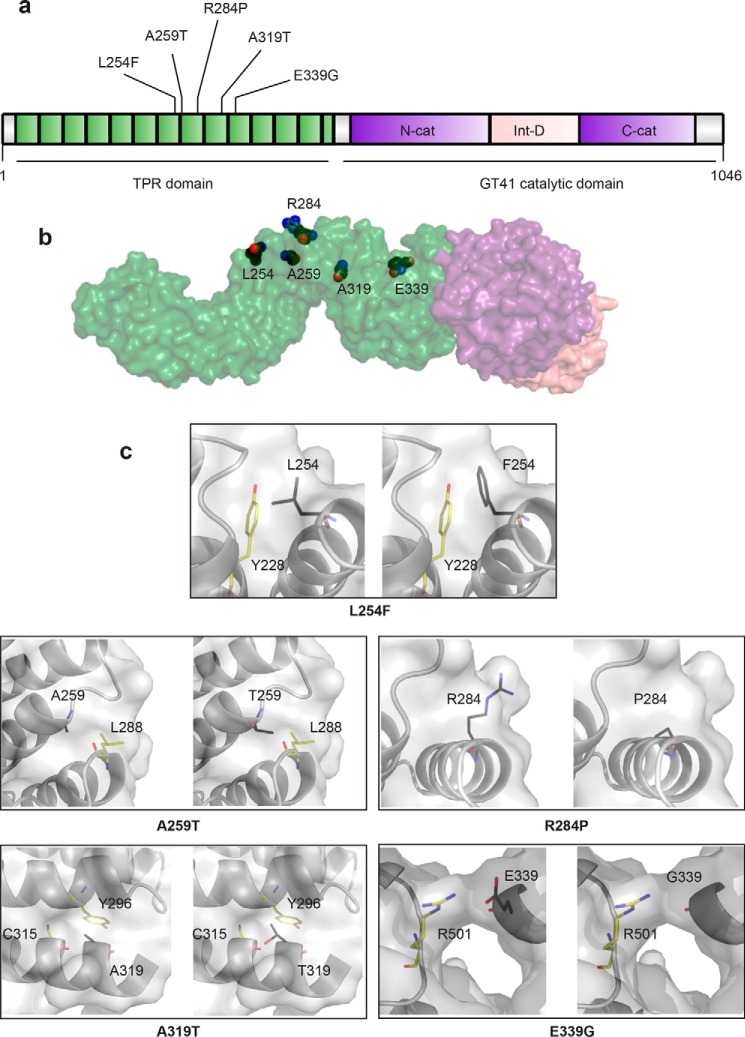
Figure 2.
All known missense mutations in the TPR domain that are causal for XLID. a, schematic of the domain organization of OGT. The N-terminal TPR domain is depicted in green with XLID variants noted, the intervening domain in pink, and the lobes of the catalytic domain in purple. b, model of full-length OGT based on the structure of the first 11.5 TPRs (PDB code 1W3B (14)) and the structure of the catalytic domain with the last 2.5 TPRs (PDB code 4AY5 (52)). The domains in the model are colored as in the schematic, and the side chains of the residues in the TPR that are mutated in XLID are depicted as spheres with black carbons. c, close-up of residues that are mutated in XLID. Panels on left show the WT residue in the amino acid position indicated, and panels on right show the replacing residue modeled into the structure (side chains are shown as sticks with black carbons). Surface representation shown in both panels is from OGT-WT. Side chains of neighboring residues that could clash are shown as sticks with yellow carbons.