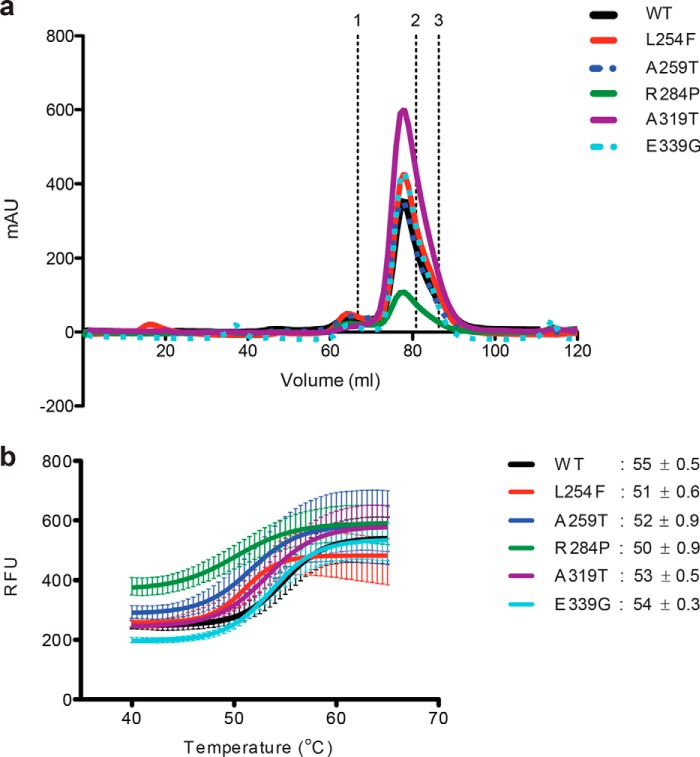
Figure 3.
OGT XLID–TPR mutants form functional dimers but demonstrate decreased thermal stability. a, TPR domain is a constitutive homodimer in solution. Samples of WT and mutant TPR domains were analyzed on a Superdex 200 size-exclusion column. The elution profiles are plotted as A280 against elution volume. Elution time of standards of known molecular weight are marked above; 1, IgG 158 kDa; 2, human albumin 66 kDa; and 3, ovalbumin 44 kDa. The His6-tagged TPR dimer has an expected molecular mass of ∼90 kDa and an apparent molecular mass of ∼120 kDa (14) due to its elongated, nonglobular structure. b, thermal denaturing curves of WT or mutant OGT TPR domains. The melting temperatures (Tm, the temperature at which both the folded and unfolded states of a protein are equally populated at equilibrium) of the proteins are indicated. Fluorescence of Sypro Orange is plotted against temperature. The data were fitted to Boltzmann sigmoidal curve equation using Prism (GraphPad). Experiments were performed in triplicate, and error bars represent mean ± S.E.