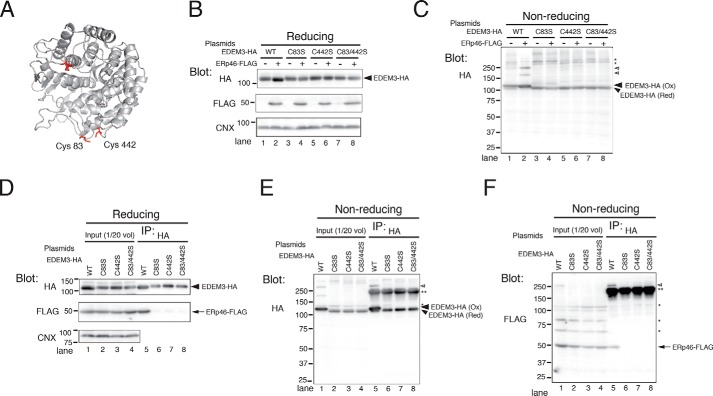
Figure 3.
ERp46 covalently associates with the mannosidase domain of EDEM3. A, ribbon model of EDEM3 mannosidase domain. The 3D structure of EDEM3 mannosidase domain (Mus musculus) was modeled based on the crystallographic structure of human ERM (Protein Data Bank code 1FMI) using SWISS-MODEL. Four Cys residues (Cys83, Cys226, Cys229, and Cys442) in the mannosidase domain are highlighted in red. B and C, redox states of EDEM3 and its Cys mutants. Cell lysates were analyzed by Western blotting under reducing (B) and nonreducing (C) conditions. Open arrowheads, complexes covalently associated with EDEM3; *, signals nonspecifically detected by the anti-HA antibody. D–F, Cys residues in the EDEM3 mannosidase domain are required for the covalent association with ERp46. EDEM3 and its Cys mutants were immunoprecipitated and electrophoresed under reducing (D) and nonreducing (E and F) conditions. Open arrowhead, complexes covalently associated with EDEM3; **, native Igs used to pull down EDEM3-HA.