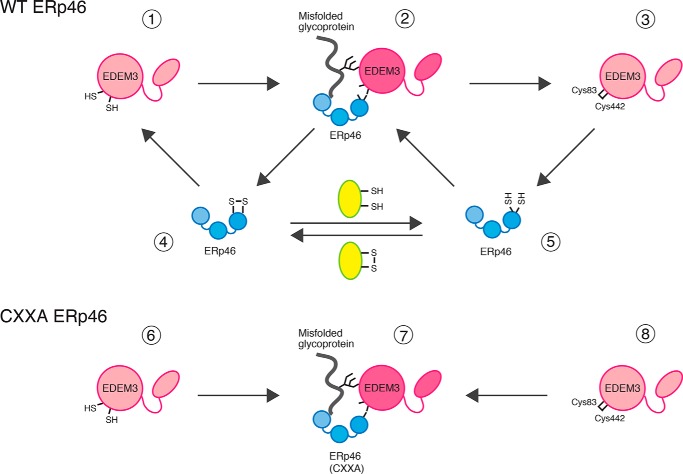
Figure 7.
Model: Redox cycle of the EDEM3-ERp46 complex, which regulates the mannose-trimming activity of EDEM3. ERp46 (4 or 5) binds to EDEM3 monomer (1 or 3) to form an intermolecular disulfide bridge between its redox-active sites and Cys83/Cys442 of EDEM3 (2) by transferring a disulfide bond to the reduced active site Cys. Covalent interaction of ERp46 with EDEM3 induces structural changes in the EDEM3 mannosidase domain, which triggers mannose-trimming activity and/or provides the scaffold for the substrate recognition. The cycle could be facilitated by another acceptor protein (yellow oval), regulating the demannosylation activity of EDEM3. In cells overexpressing CXXA mutant of ERp46 (bottom), disulfide bond of the complex (7) cannot be transferred owing to the lack of isomerase activity, leading to the depletion of EDEM3 monomer (6 or 8). ERp46 has three redox-active sites, but only one Cys pair is shown in the cartoon.