Abstract
Gliomas are genetically and histopathologically heterogeneous. Intratumoral heterogeneity in the MGMT promoter methylation status is an important clinical biomarker of glioblastoma. A higher uptake of 11C-methionine in positron-emission tomography (PET) reportedly reflects increased MGMT promoter methylation; however, non-stereotactic comparison of MGMT methylation and 11C-methionine PET images may not be accurate. The present study examined the correlation between 11C-methionine uptake and MGMT promoter methylation in non-enhancing gliomas using stereotactic image-based histological analysis. Data were collected from 9 patients with newly diagnosed non-enhancing glioma who underwent magnetic resonance imaging and 11C-methionine PET during pre-surgical examination. Clinical data were also collected from 3 patients during repeat surgery. The correlation between 11C-methionine uptake and MGMT methylation or cell density was analyzed using histological specimens obtained by multiple stereotactic sampling and an exact local comparison of 11C-methionine PET images and histological specimens was made. A total of 31 stereotactic sample sites were identified. In newly diagnosed cases, the tumor to normal uptake (T/N) ratio revealed a significant positive correlation with MGMT methylation (R=0.54, P=0.009) and a marginal correlation with cell density (R=0.42, P=0.05). In recurrent cases, the T/N ratio demonstrated no correlation with MGMT methylation (R=0.01, P=0.97) or cell density (R=0.15, P=0.70). An increased uptake of 11C-methionine in PET may reflect increased MGMT promoter methylation according to stereotactic image-based histological analysis. 11C-methionine PET could therefore be a useful tool for detecting regional MGMT promoter methylation in non-enhancing primary glioma.
Keywords: 11C-methionine positron emission tomography, glioma, MGMT promoter methylation
Introduction
MGMT promoter methylation is associated with a favorable outcome after temozolomide chemotherapy in patients with newly diagnosed glioblastoma (1). Furthermore, temozolomide rechallenge is a treatment option for recurrent MGMT promoter-methylated glioblastoma (2). Evaluation of MGMT methylation status is thus important for treatment of primary and recurrent glioma.
Gliomas are genetically and histopathologically heterogeneous (3–5). A previous study has demonstrated intratumoral heterogeneity in MGMT promoter methylation status (6). Therefore, it is doubtful that a single biopsy specimen can represent the molecular landscape of the entire tumor.
MGMT promoter methylation status can change between the first surgery for newly diagnosed glioblastoma and a second surgery for recurrent disease (7,8). The development of reduced methylation in the MGMT promoter leads to acquired therapeutic resistance after temozolomide treatment in glioblastomas (9). Re-evaluation of MGMT promoter methylation status at the time of recurrence is therefore important for selecting treatment.
Attempts to assess gene mutations non-invasively by imaging technology have been conducted in the past, but there are few reports on image evaluation of glioma-related gene mutations (10–15). However, among these, 11C-methionine positron-emission tomography (11C-methionine PET) has been proven to be a useful tool for detecting MGMT promoter methylation in non-enhancing glioma (12). Nevertheless, given the heterogeneity of MGMT promoter methylation status in gliomas, a non-stereotactic comparison of MGMT promoter methylation and 11C-methionine uptake in PET images may not be accurate.
In this study, the correlation between 11C-methionine PET and MGMT promoter methylation status in non-enhancing gliomas was stereotactically verified.
Materials and methods
Subjects and glioma tumor samples
The present study was performed in accordance with the principles of the Helsinki Declaration, and approval for this study was obtained from the ethical committee of Osaka National Hospital (no. 94, IRB no. 0713). All patients provided written informed consent.
Clinical data were collected from 9 patients with newly diagnosed glioma, who underwent both magnetic resonance imaging (MRI) and 11C-methionine PET as part of their pre-surgical examination, from 2014 to 2017. Clinical data were also collected from 3 of 9 patients with newly diagnosed glioma during surgery for recurrent disease. Recurrent cases received chemotherapy or chemoradiotherapy prior to 11C-methionine PET examination. Tumor tissue specimens were also obtained from each patient. Multiple tissue sites were stereotactically sampled, and each sample was evenly divided. One part of each sample was subjected to pathological examination and the other was subjected to MGMT methylation status analysis.
Histopathological analysis
A subset of the tumor samples was fixed in 10% formalin and embedded in paraffin wax following standard procedures. In each case, hematoxylin and eosin (H&E)-stained sections were examined to classify the tumors according to the World Health Organization (WHO) International Histological Classification of Tumors. Cell counting was performed under a light microscope (Olympus Corporation, Tokyo, Japan; ×200 magnification) and data were recorded as the mean of 3 different locations within the specimen according to the method used in our previous study (16).
Genomic DNA Extraction
Tumor samples were immediately frozen in liquid nitrogen and stored at −80°C. Genomic DNA was extracted using the DNeasy Blood and Tissue kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer's protocol (12).
MGMT Promoter Methylation Analysis
MGMT promoter methylation status was determined by quantitative methylation-specific PCR (qMSP) as described in our previous study (12). DNA extracted from tumor tissue was subjected to bisulfite modification by an EZ DNA Methylation-Gold kit (Zymo Research Corp., Irvine, CA, USA), according to the manufacturer's instructions. Bisulfite-modified DNA was analyzed by qMSP using the QuantStudio™ 12K Flex Real-Time PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with POWER SYBR® Green PCR Master Mix (Thermo Fisher Scientific, Inc.). Methylated and unmethylated DNA molecules were amplified separately using specific primers (17). Quantification of methylated and unmethylated sequences was performed by employing the standard curve method, using serial dilutions of bisulfite-modified EpiScope® Methylated HCT116 gDNA (Takara Bio, Inc., Shiga, Japan), which is highly methylated by CpG methylase, and EpiScope® Unmethylated HCT116 DKO gDNA (Takara Bio, Inc.), obtained from cells that genetically lack both DNA methyltransferase 1 and DNA methyltransferase 3B. The percentages of methylation and standard deviations (S.D.) were calculated from triplicate PCRs.
11C-methionine PET
PET images were obtained using a SET-3000 GCT/X scanner (Shimadzu Corp., Kyoto, Japan) with gadolinium oxyorthosilicate crystals as emission detectors. The 11C-methionine tracer was synthesized in accordance with the method described by Berger et al (18) and injected intravenously at a dose of 111-222 mBq (3–6 mCi). Tracer accumulation was recorded over 15 min in 99 transaxial slices, spanning the entire brain. The summed activity at 20-35 min after tracer injection was used for image reconstruction. Images were stored in 256×256×99 anisotropic voxels, with a voxel size of 1×1×2.6 mm. The tumor/normal tissue (T/N) ratios were calculated stereotactically by dividing the standard uptake value (SUV) for the tumor by the SUV of the contralateral lesion in the same way as in our previous study (16).
Magnetic resonance imaging
All patients were studied using a 1.5 T whole-body MR system (Achieva; Philips Healthcare, Amsterdam, The Netherlands) within a week before the operation. T1-weighted imaging with gadolinium enhancement was used to select patients with non-enhancing gliomas. T2-weighted (T2) or FLAIR images were acquired in all cases for delineation of tumors.
Image fusion and registration
We used the Brainlab VectorVision compact neuronavigation system (Brainlab, Munich, Germany). This neuronavigation system is composed of a surgical planning workstation with software tools for coregistration of multimodal image sets. The PET image was registered on fluid-attenuated inversion recovery (FLAIR) standard anatomical MRI images using the Brainlab VectorVision compact neuronavigation system. These registered images were transferred to the navigation system for stereotactic surgery.
Surgery for stereotactic multiple sampling evaluation
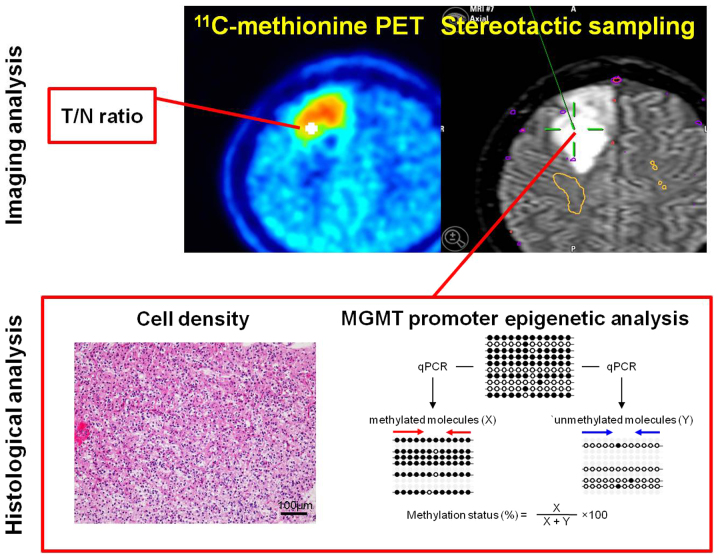
We used a stereotactic multiple sampling evaluation for gliomas as previously described (16). The location for tumor biopsy was preoperatively determined on FLAIR images and 11C-methionine PET. 3D gapless FLAIR images and PET data, co-registered beforehand, were transferred to the Brainlab VectorVision compact neuronavigation system and the biopsy target for histopathological examination was planned (Fig. 1). Standard craniotomy was performed under general anesthesia. Multiple sampling biopsy was performed in non-enhancing tumor lesions targeted for resection by inserting a catheter, aimed at the target, immediately after craniotomy, in order to minimize the error caused by brain shifting. Although multiple tissue sampling was performed in some cases, real-time navigation was performed to confirm the position of each biopsy site within the tumor.
Figure 1.
Illustration of the stereotactic sampling method and histological analysis. The sampling site is recorded on the neuronavigation image, which is a fused 11C-methionine PET/magnetic resonance image. Cell density and MGMT methylation are analyzed at precisely the same position in each sampling specimen. PET, positron emission tomography.
Statistical analysis
All data are presented as the mean value unless otherwise stated. Statistical analysis was performed using JMP version 8 (SAS Institute, Inc., Cary, NC, USA). A linear regression model, using the method for least squares, was used for modeling 2 or 3 independent variables. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
Detailed information on the 9 investigated patients is listed in Table I. Tissue specimens at 31 sampling sites were stereotactically obtained using an intraoperative neuronavigation system. All patients had non-enhancing lesions on MRI.
Table I.
Caracteristics of patients with non-enhancing glioma.
| Patient no. | Age (years) | Sex | Histologic diagnosis | Primary or recurrent | Treatment before operation | Localization | No. of specimens | T/N ratio | MGMT methylation (%) | MGMT methylation status (M: ≥3%, U:<3%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 57 | F | Diffuse astrocytoma | Primary | None | R frontal | 1 | 1.19 | 48.48 | M |
| 2 | 1.33 | 46.51 | M | |||||||
| 3 | 1.32 | 17.5 | M | |||||||
| 1–2 | 59 | F | Glioblastoma | First recurrent | TMZ | L frontal | 1 | 1.11 | 2.77 | U |
| 2 | 1.45 | 12.91 | M | |||||||
| 2–1 | 79 | M | Diffuse astrocytoma | Primary | None | R parietal | 1 | 1.24 | 0.06 | U |
| 2 | 1.06 | 0.03 | U | |||||||
| 3 | 2.23 | 0.17 | U | |||||||
| 2–2 | 81 | M | Anaplastic astrocytoma | First recurrent | TMZ | R temporal | 1 | 0.96 | 0.38 | U |
| 2 | 1.49 | 0.24 | U | |||||||
| 2–3 | 81 | M | Anaplastic astrocytoma | Second recurrent | RT + TMZ | L parietal | 1 | 1.82 | 0.24 | U |
| 2 | 1.52 | 0.2 | U | |||||||
| 3 | 66 | M | Oligodendroglioma | Primary | None | L frontal | 1 | 1.01 | 19.77 | M |
| 2 | 1.34 | 8.16 | M | |||||||
| 4 | 43 | M | Oligodendroglioma | Primary | None | L frontal | 1 | 1.38 | 2.56 | U |
| 2 | 1.22 | 2.29 | U | |||||||
| 3 | 1.29 | 2.61 | U | |||||||
| 5–1 | 46 | F | Oligodendroglioma | Primary | None | R frontal | 1 | 1.96 | 47.93 | M |
| 2 | 2.45 | 84.67 | M | |||||||
| 5–2 | 42 | F | Anaplastic oligodendroglioma | First recurrent | TMZ | R frontal | 1 | 1.08 | 0.32 | U |
| 2 | 0.59 | 1.34 | U | |||||||
| 3 | 2.03 | 0.62 | U | |||||||
| 6 | 31 | F | Anaplastic astrocytoma | Primary | None | R frontal | 1 | 1.03 | 1.6 | U |
| 2 | 1.39 | 4.75 | M | |||||||
| 3 | 0.95 | 1.39 | U | |||||||
| 7 | 38 | M | Anaplastic astrocytoma | Primary | None | L frontal | 1 | 1.56 | 0.14 | U |
| 2 | 1.42 | 0.21 | U | |||||||
| 3 | 0.73 | 0.23 | U | |||||||
| 8 | 58 | F | Glioblastoma | Primary | None | L temporal | 1 | 1.25 | 0.21 | U |
| 9 | 50 | M | Glioblastoma | Primary | None | R occipital | 1 | 1.17 | 0.2 | U |
| 2 | 1.16 | 0.33 | U |
M, male; F, female; R, right; L, left; RT, radiotherapy; TMZ, temozolomide; M, methylated; U, unmethylated; MGMT, O(6)-methylguanine-DNA methyltransferase; T/N, tumor to normal uptake ratio.
Intratumoral heterogeneity in MGMT promoter methylation status
In our previous report, we evaluated the correlation between T/N ratio and MGMT methylation in a non-stereotactic manner, and found that a threshold T/N ratio value of 1.6 significantly correlated with a quantitative threshold MGMT methylation status of 3% (12). Using a positive methylation assay threshold of 3, 22.2% (2 of 9) of cases in this study demonstrated intratumoral heterogeneity in terms of methylation levels, with the percentage of methylation varying up to 4.66-fold for each case (Table I).
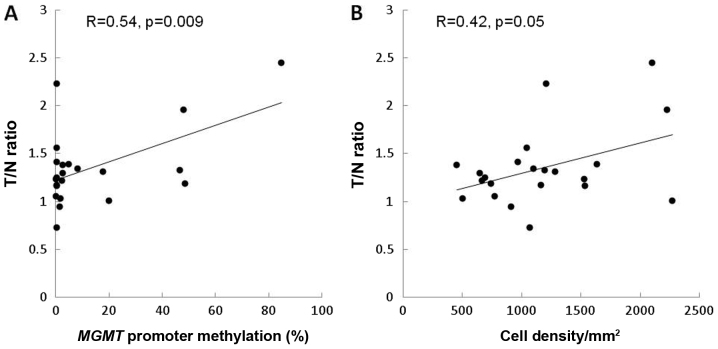
Correlation of PET T/N 11C-methionine ratio with MGMT methylation and cell density in stereotactic image-based histological comparisons. In newly diagnosed cases, the T/N ratio correlated positively with MGMT methylation (R=0.54, P=0.009) (Fig. 2A). In contrast, the T/N ratio showed a marginal correlation with cell density (R=0.42, P=0.05) (Fig. 2B).
Figure 2.
Correlation of the T/N ratio of 11C-methionine positron emission tomography with MGMT methylation and cell density in a histological comparison, using stereotactic images of primary gliomas. (A) The T/N ratio reveals a positive correlation with MGMT methylation (R=0.54, P=0.009). (B) The T/N ratio shows a marginal correlation with cell density (R=0.42, P=0.05). T/N ratio, tumor to normal uptake ratio.
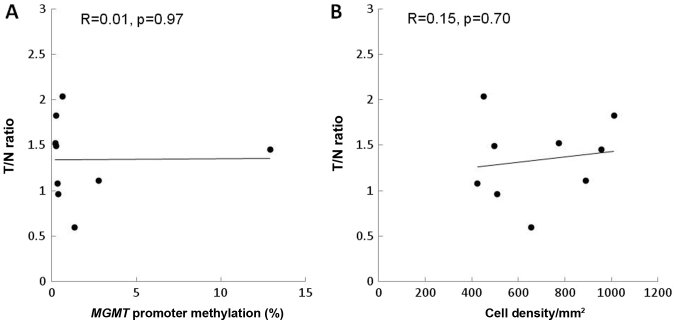
In recurrent cases, the T/N ratio did not correlate with MGMT methylation (R=0.01, P=0.97) or with cell density (R=0.15, P=0.70) (Fig. 3A and B).
Figure 3.
Correlation of the T/N ratio of 11C-methionine positron emission tomography with MGMT methylation and cell density in a histological comparison using stereotactic images of recurrent gliomas. The T/N ratio revealed no correlation with (A) MGMT methylation (R=0.01, P=0.97) or (B) cell density (R=0.15, P=0.7).
Estimation of 11C-methionine PET with MGMT methylation and cell density
We attempted to model the PET T/N ratio of 11C-methionine according to MGMT methylation and cell density in newly diagnosed cases. A linear regression model indicated the following equation, with an overall P-value of 0.025, which was considered statistically significant for fit.
(T/Nr of 11C-methionie PET)=0.0002×CD+0.004×MGMT+1.1,
where CD is the cell density (cells/mm2) and MGMT indicates MGMT methylation (%). Multiple regression analysis revealed that MGMT methylation tended to be statistically significant for model construction (t=2.03, P=0.057), but cell density was not (t=0.87, P=0.4).
Discussion
Previously, we evaluated the highest T/N ratio of 11C-methionine PET in non-enhancing gliomas, in a non-stereotactic manner, and identified that a higher uptake of 11C-methionine in PET may reflect increased MGMT promoter methylation (12). However, gliomas are genetically and histopathologically heterogeneous (3–5). A previous report had demonstrated intratumoral heterogeneity in MGMT promoter methylation status in 14% of cases, with the percentage of methylation varying up to 4-fold within each case, based on pyrosequencing results (6). Thus, caution must be taken when interpreting the results of genomic biomarker analyses based on a single biopsy specimen from a given tumor. Therefore, in this study, we used stereotactic image-based histological analysis and identified the correlation between the T/N ratio and MGMT promoter methylation in multiple, spatially distinct samples of primary gliomas. Using qMSP, we found that 22.2% of cases demonstrated intratumoral heterogeneity in methylation levels.
11C-methionine uptake is mainly determined by a sodium-independent L-transporter system in the luminal membrane of endothelial cells (19–21) and correlates with the proliferation and microvessel density of tumors (16,18,22,23). Although the main metabolic pathway of 11C-methionine is protein incorporation, the conversion of methionine to S-adenosyl-L-methionine (SAM) is a minor pathway (24). In the brain, methionine is a precursor of SAM (25) which plays an important role in DNA methylation processes (26). The activation of methionine to SAM apparently occurs very rapidly in the brain, as measured by the conversion of administered 11C-methionine, and 11C-SAM uptake may reflect the enhanced transmethylation processes in tumors (24,27). Methionine uptake in tumors may be associated with MGMT promoter methylation by transmethylation.
In this study, the T/N ratio correlated positively with the MGMT methylation rate in primary gliomas, but not in recurrent tumors. Various authors have assessed changes in MGMT promoter methylation status in paired initial and recurrent glioblastoma samples (7,8). Selective survival of tumor cells with high MGMT expression during alkylating agent therapy may lead to a change in MGMT status at recurrence (7,21,28).
In our previous study, we suggested that cell density in glioma tissue contributes to 11C-methionine uptake in stereotactic analysis (16). Furthermore, in another series of patients, including those with high grade gliomas, no statistically significant difference in T/N 11C-methionine uptake ratios were found between patients with and without MGMT methylation (29,30). Methionine uptake in gliomas is governed by changes in tracer influx across the blood-brain barrier (BBB) (31). In previous studies (16,29,30), the enhancing gliomas and methionine uptake were affected by BBB disruption and reflected the amino acid transport system and proliferation. Our present results analyzed non-enhancing gliomas and suggested that MGMT methylation tends to contribute markedly more to 11C-methionine uptake than to cell density in glioma tissue. However, the linear correlation of MGMT methylation and 11C-methionine uptake mainly arose from oligodendroglioma cases. An evaluation of the grading and histological types of gliomas and biopsy sites should be performed from this perspective.
This study was limited by its small sample size. Moreover, it remains unclear whether our results will be useful for prognosis and predicting response to therapy. Future large-scale studies are required to validate the proposed correlation between MGMT methylation and 11C-methionine uptake in a stereotactic manner.
To the best of our knowledge, no previous study has demonstrated stereotactic imaging of MGMT methylation status in gliomas using the noninvasive 11C-methionine PET imaging technique. When we investigated the correlation of the 11C-methionine T/N ratio in PET with quantitative MGMT promoter methylation for non-enhancing gliomas in a stereotactic image-based histological analysis, we found that the T/N ratio correlated positively with the MGMT methylation rate in primary gliomas. Our findings can contribute to understanding of the local characteristics of MGMT methylation status in primary and recurrent gliomas.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- FLAIR
fluid-attenuated inversion recovery
- MRI
magnetic resonance imaging
- PET
positron-emission tomography
- qMSP
quantitative methylation-specific PCR
- SUV
standard uptake value
- T/N ratio
tumor/normal tissue ratio
- WHO
World Health Organization
Funding
This work was supported by the JSPS KAKENHI (grant nos. JP15K15534 and 16K20033).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YO was responsible for the conception of the study and its design. YO and MN acquired the data. YO, MK, TS, DK, EY, YKo, MM and YKa, SN and TF analyzed and interpreted the data. YO drafted the manuscript. All authors critically revised the manuscript for important intellectual content and read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Osaka National Hospital (approval no. 94; IRB no. 0713). All patients provided written informed consent prior to their inclusion within the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 2.Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, Schnell O, Hau P, Herrlinger U, Sabel MC, et al. MGMT Promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: The DIRECTOR trial. Clin Cancer Res. 2015;21:2057–2064. doi: 10.1158/1078-0432.CCR-14-2737. [DOI] [PubMed] [Google Scholar]
- 3.Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH. Molecular and cellular heterogeneity: The hallmark of glioblastoma. Neurosurg Focus. 2014;37:E11. doi: 10.3171/2014.9.FOCUS14521. [DOI] [PubMed] [Google Scholar]
- 4.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: A social network. Cancer Res. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja R, Sinha N, Saini J, Mahadevan A, Rao KN, Swaminathan A. Assessment of tissue heterogeneity using diffusion tensor and diffusion kurtosis imaging for grading gliomas. Neuroradiology. 2016;58:1217–1231. doi: 10.1007/s00234-016-1758-y. [DOI] [PubMed] [Google Scholar]
- 6.Parker NR, Hudson AL, Khong P, Parkinson JF, Dwight T, Ikin RJ, Zhu Y, Cheng ZJ, Vafaee F, Chen J, et al. Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci Rep. 2016;6:22477. doi: 10.1038/srep22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes AA, Franceschi E, Tosoni A, Bartolini S, Bacci A, Agati R, Ghimenton C, Turazzi S, Talacchi A, Skrap M, et al. O(6)-methylguanine DNA-methyltransferase methylation status can change between first surgery for newly diagnosed glioblastoma and second surgery for recurrence: Clinical implications. Neuro Oncol. 2010;12:283–288. doi: 10.1093/neuonc/nop050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkinson JF, Wheeler HR, Clarkson A, McKenzie CA, Biggs MT, Little NS, Cook RJ, Messina M, Robinson BG, McDonald KL. Variation of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation in serial samples in glioblastoma. J Neurooncol. 2008;87:71–78. doi: 10.1007/s11060-007-9486-0. [DOI] [PubMed] [Google Scholar]
- 9.Park CK, Kim JE, Kim JY, Song SW, Kim JW, Choi SH, Kim TM, Lee SH, Kim IH, Park SH. The changes in MGMT promoter methylation status in initial and recurrent glioblastomas. Transl Oncol. 2012;5:393–397. doi: 10.1593/tlo.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4:116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okita Y, Nonaka M, Shofuda T, Kanematsu D, Yoshioka E, Kodama Y, Mano M, Nakajima S, Kanemura Y. (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin Neurol Neurosurg. 2014;125:212–216. doi: 10.1016/j.clineuro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107:197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito T, Maruyama T, Muragaki Y, Tanaka M, Nitta M, Shinoda J, Aki T, Iseki H, Kurisu K, Okada Y. 11C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am J Neuroradiol. 2013;34:85–91. doi: 10.3174/ajnr.A3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinozaki N, Uchino Y, Yoshikawa K, Matsutani T, Hasegawa A, Saeki N, Iwadate Y. Discrimination between low-grade oligodendrogliomas and diffuse astrocytoma with the aid of 11C-methionine positron emission tomography. J Neurosurg. 2011;114:1640–1647. doi: 10.3171/2010.11.JNS10553. [DOI] [PubMed] [Google Scholar]
- 16.Okita Y, Kinoshita M, Goto T, Kagawa N, Kishima H, Shimosegawa E, Hatazawa J, Hashimoto N, Yoshimine T. (11)C-methionine uptake correlates with tumor cell density rather than with microvessel density in glioma: A stereotactic image-histology comparison. Neuroimage. 2010;49:2977–2982. doi: 10.1016/j.neuroimage.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- 18.Berger G, Maziere M, Knipper R, Prenant C, Comar D. Automated synthesis of 11C-labelled radiopharmaceuticals: Imipramine, chlorpromazine, nicotine and methionine. Int J Appl Radiat Isot. 1979;30:393–399. doi: 10.1016/0020-708X(79)90049-8. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen GM, Pettigrew KD, Patlak CS, Hertz MM, Paulson OB. Asymmetrical transport of amino acids across the blood-brain barrier in humans. J Cereb Blood Flow Metab. 1990;10:698–706. doi: 10.1038/jcbfm.1990.123. [DOI] [PubMed] [Google Scholar]
- 20.Souba WW, Pacitti AJ. How amino acids get into cells: Mechanisms, models, menus, and mediators. JPEN J Parenter Enteral Nutr. 1992;16:569–578. doi: 10.1177/0148607192016006569. [DOI] [PubMed] [Google Scholar]
- 21.Christmann M, Nagel G, Horn S, Krahn U, Wiewrodt D, Sommer C, Kaina B. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: A comparative study on astrocytoma and glioblastoma. Int J Cancer. 2010;127:2106–2118. doi: 10.1002/ijc.25229. [DOI] [PubMed] [Google Scholar]
- 22.Bergström M, Lundqvist H, Ericson K, Lilja A, Johnström P, Långström B, von Holst H, Eriksson L, Blomqvist G. Comparison of the accumulation kinetics of L-(methyl-11C)-methionine and D-(methyl-11C)-methionine in brain tumors studied with positron emission tomography. Acta Radiol. 1987;28:225–229. doi: 10.3109/02841858709177339. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, Yoshimura S, Maruyama T, Muragaki Y, Iwama T. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol. 2008;29:1176–1182. doi: 10.3174/ajnr.A1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishiwata K, Ido T, Abe Y, Matsuzawa T, Iwata R. Tumor uptake studies of S-adenosyl-L-[methyl-11C]methionine and L-[methyl-11C]methionine. Int J Rad Appl Instrum B. 1988;15:123–126. doi: 10.1016/0883-2897(88)90077-3. [DOI] [PubMed] [Google Scholar]
- 25.Baldessarini RJ, Kopin IJ. S-adenosylmethionine in brain and other tissues. J Neurochem. 1966;13:769–777. doi: 10.1111/j.1471-4159.1966.tb09884.x. [DOI] [PubMed] [Google Scholar]
- 26.Andreoli VM, Agnoli A, Fazio C. Transmethylations and the central nervous system. Springer-Verlag; Berlin; 1978. [DOI] [PubMed] [Google Scholar]
- 27.Ishiwata K, Vaalburg W, Elsinga PH, Paans AM, Woldring MG. Comparison of L-[1-11C]methionine and L-methyl-[11C]methionine for measuring in vivo protein synthesis rates with PET. J Nucl Med. 1988;29:1419–1427. [PubMed] [Google Scholar]
- 28.Wiewrodt D, Nagel G, Dreimüller N, Hundsberger T, Perneczky A, Kaina B. MGMT in primary and recurrent human glioblastomas after radiation and chemotherapy and comparison with p53 status and clinical outcome. Int J Cancer. 2008;122:1391–1399. doi: 10.1002/ijc.23219. [DOI] [PubMed] [Google Scholar]
- 29.Choi H, Bang JI, Cheon GJ, Kim YH, Park CK, Park SH, Kang KW, Chung JK, Kim EE, Lee DS. 18F-fluorodeoxyglucose and11C-methionine positron emission tomography in relation to methyl-guanine methyltransferase promoter methylation in high-grade gliomas. Nucl Med Commun. 2015;36:211–218. doi: 10.1097/MNM.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 30.Lopci E, Riva M, Olivari L, Raneri F, Soffietti R, Piccardo A, Bizzi A, Navarria P, Ascolese AM, Rudà R, et al. Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging. 2017;44:1155–1164. doi: 10.1007/s00259-017-3618-3. [DOI] [PubMed] [Google Scholar]
- 31.Roelcke U, Radü EW, von Ammon K, Hausmann O, Maguire RP, Leenders KL. Alteration of blood-brain barrier in human brain tumors: Comparison of [18F]fluorodeoxyglucose, [11C]methionine and rubidium-82 using PET. J Neurol Sci. 1995;132:20–27. doi: 10.1016/0022-510X(95)00117-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.