Abstract
The invasiveness of glioma cells is the predominant clinical problem associated with this tumor type, and is correlated with pathological malignant grade. ZEB1 is highly expressed in glioma cells and associated with the aggressiveness of various types of cancer. In the present study, the expression of ZEB1 and ZEB2 was examined with the aim of determining the role of ZEBs in glioma. ZEB1 and ZEB2 were highly expressed in all glioma cells used in this study. Double knockdown of ZEB1 and ZEB2 suppressed tumor invasiveness more effectively than knockdown of either alone, in both in vitro and in vivo experiments. ZEB1 and ZEB2 were marginally expressed in grade II, but expressed at higher levels in grade IV. Importantly, ZEB-positive cells were more abundant in recurrent glioma with malignant transformation than in initial grade II tissue from the same case. These results indicate that the levels of ZEB1 and ZEB2 are positively correlated with histopathological grade and invasiveness of glioma, suggesting that δEF1 family proteins (ZEB1 and ZEB2) could be useful as prognostic markers and therapeutic targets in patients with glioma.
Keywords: glioma, ZEB1 (δEF1), ZEB2 (SIP1), gene silencing, invasion
Introduction
Gliomas are the most common primary intra-axial brain tumor in adults. According to the WHO classification of tumors in the central nervous system (1), glioma is classified into four grades based on histopathological features. Median overall survival remains about 5–10 years for grade II, 2–3 years for grade III, and 12–15 months for grade IV gliomas (2). In particular, grade IV glioma has an extremely poor prognosis due to its strong tendency to infiltrate adjacent brain tissue and low response to chemoradiotherapy. Grade IV tumors include glioblastomas with mesenchymal-like features, which originate from progenitor or stem cells in the astrocytic lineage (1). In addition, when gliomas with non-mesenchymal features recur, their cellular characteristics are altered to exhibit typical mesenchymal features. A shift towards the mesenchymal subtype appears to be a common pattern in disease progression of various cancers, analogous to cancer cells undergoing the epithelial-to-mesenchymal transition (EMT).
The EMT is involved in differentiation of immature embryos, wound healing, and tumor metastasis (3,4). In most cases, the EMT is regulated by several transcription factors, including the δEF1 family of two-handed zinc-finger factors (ZEB1/δEF1 and ZEB2/SIP1). Several extracellular signaling molecules, including fibroblast growth factor (FGF)-2 and tumor necrosis factor (TNF)-α, as well as intracellular signaling molecules such as Ras, cooperate with TGF-β to upregulate ZEBs and promote the EMT (5,6). The EMT induced by TGF-β and FGF-2 specifically causes upregulation of integrin α3, which is positively correlated with aggressiveness of breast cancer cells (7). Previously, we reported that integrin α3 and laminin are highly expressed in and promote invasiveness of glioma cells, in which basal levels of ERK1/2 phosphorylation status are extremely high. Importantly, when ERK1/2 in glioma cells are inactivated by the inhibitor U0126, expression of integrin α3 and δEF1 family proteins (ZEB1 and ZEB2) is downregulated (7). Moreover, a neutralizing antibody targeting integrin α3 inhibits motile properties (8). Thus, aggressiveness in glioma appears to be correlated with EMT phenotypes.
In this study, we found that ZEB1 and ZEB2 were expressed at high levels in glioma cells. Although single knockdown of either ZEB1 or ZEB2 alone moderately inhibited invasion and anchorage-independent growth, double knockdown of both proteins had more pronounced effects. Glioma cells in which both ZEB1 and ZEB2 were silenced, formed smaller tumors in mice. Immunohistochemical (IHC) analyses using human surgical specimens revealed that ZEB1 and ZEB2 were more highly expressed in specimens of grade IV than grade II glioma. Importantly, ZEB-positive cells were more abundant in specimens from patients with recurrent glioma, suggesting that both ZEB1 and ZEB2 are overexpressed in glioma with more aggressive phenotypes. Thus, δEF1 family proteins represent promising prognostic markers for glioma.
Materials and methods
Cell culture and antibodies
MCF-7, MDA-MB-231, and A172 cells were from the American Type Culture Collection (Manassas, VA, USA). KG-1-C, U251, and T98G cells were from Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan). GL261 cells was from Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin (Nacalai Tesque), and 100 µg/ml streptomycin (Nacalai Tesque). Mouse monoclonal anti-α-tubulin antibody was purchased from Sigma-Aldrich. Rabbit polyclonal anti-ZEB1 and -ZEB2 antibodies were from Novus Biologicals (Littleton, CO, USA).
RNA interference, RNA isolation, and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Transfection of siRNA was performed according to the protocol recommended for RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). siRNAs and shRNAs against ZEB1 and ZEB2 were also described previously (6,7). Total RNA was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and used for RT-qPCR analyses. RT-qPCR analysis was performed using the Power SYBR Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). mRNA levels were normalized against the corresponding levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The primer sequences were described previously (6).
Immunoblotting
The procedures used for immunoblotting were previously described (6). Immunodetection was performed with the ECL blotting system (Amersham Bioscience, Piscataway, NJ, USA) on a Luminescent Image Analyzer (LAS4000; Fujifilm, Tokyo, Japan). α-tubulin was used as a loading control.
Cell invasion, proliferation and colony formation assays
Procedures were as previously described (6,7,9). For the invasion assay, cells were photographed and visually counted, and the cell counts were subjected to statistical analyses. For proliferation assays, 24 h after infection or transfection, cells were trypsinized, counted, and re-seeded in triplicate in 96-well plates. Four days later, Cell Count Reagent SF (Nacalai Tesque) was used according to the recommended protocol, and the results were evaluated by statistical analyses. For colony formation assays, cells in 0.3% agar (Nacalai Tesque) were covered with culture media for 3 weeks. Cell viability was measured using Cell Count Reagent SF, which was added to the media and incubated for 60 min. An aliquot was taken and colorimetrically quantified at 450–650 nm.
Allo/Xenograft glioma implants in mouse brain
All experimental animals were reared in accordance with the animal experiment protocols approved by University of Yamanashi's Administrative Panel on Laboratory Animal Models. Five-week-old male mice were used for intracranial model of tumor cells implantation. Intraperitoneally anesthetized mice were set in a stereotactic frame (NARISHIGE Type SR5N), the scalp was linearly incised, and a burr hole 1 mm in diameter was introduced 3 mm lateral and 1 mm anterior from bregma. Tumor cells (1.0×105) were stereotactically transplanted at a depth of 3.5 mm under cranial bone using a Hamilton syringe. The syringe was pulled out slowly over the course of 1 min to avoid a rapid change in intracranial pressure. The burr hole was covered with sterile wax, followed by scalp suture (10). All the animal experiments were conducted in compliance with the protocol which was reviewed by the Institutional Animal Care and Use Committee and approved by the president of Yamanashi University (A24-70).
IHC analyses using human glioma specimens
Thirty-two surgical specimens were prepared from 25 glioma patients treated at Yamanashi University Hospital from 1985 to 2016. Informed consent was obtained from all subjects. Of the 32 specimens, 19 were from patients with only primary tumor, 10 were from patients who had undergone two surgical operations due to tumor recurrence, and three specimens were from a patient who had undergone three surgeries. The specimens were chosen according to the following criteria: i) no preoperative chemotherapy or irradiation before recurrence of glioma, and ii) definitive diagnosis (grade II, III, or IV) by pathologists. Intensity of ZEB1- and ZEB2-positive cells was scored: 0 for negative (fewer than 1% positive cells in a field); 1 for weak (1 to 24%); 2 for intermediate (25 to 49%); and 3 for strongly positive (more than 50%). All studies were conducted using the protocol approved by the Ethics Committee of the University of Yamanashi (1642).
Statistical analysis
Survival analyses of implanted mice were performed using Kaplan-Meier curves and analyzed using log-rank test. One-way factorial ANOVA followed by Fisher's least significant difference (PLSD) test was used to compare means among multiple groups. Non-parametric Mann-Whitney U tests and Student's t-tests were used to compare means between two groups. Spearman's rank correlation coefficient tests were used to identify significant correlations between ZEB1 and ZEB2. IBM SPSS statistics version 22 software (IBM Japan, Tokyo, Japan) was used for all statistical analyses.
Results
Anti-tumor effects of ZEB siRNAs in mouse glioma
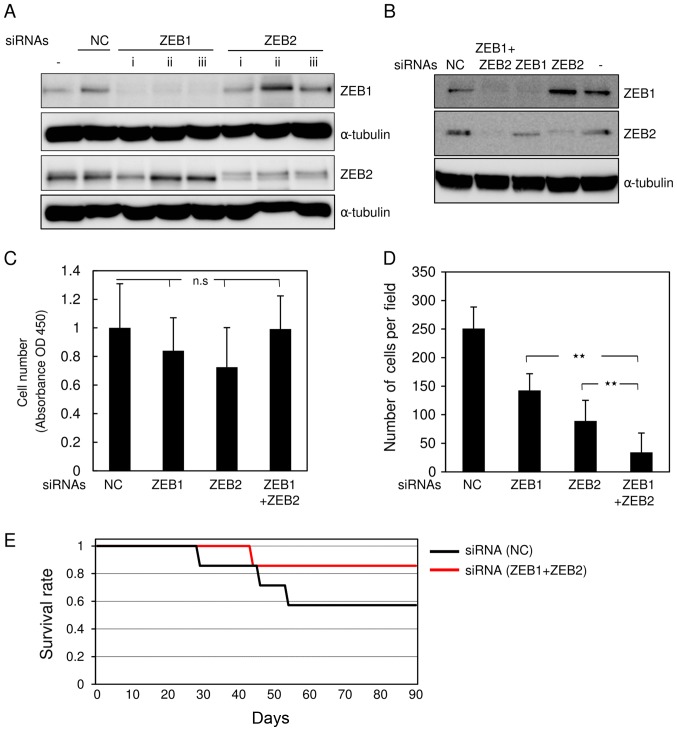
We reported previously that ZEB1 and ZEB2 are highly expressed and function redundantly in breast cancer cells (6). To investigate the roles of these factors in glioma, we simultaneously knocked down both ZEB1 and ZEB2 in mouse glioma GL261 cells. For this purpose, the cells were transfected with siRNAs against mouse ZEB1 and ZEB2. All siRNAs effectively silenced the corresponding genes when transfected alone (Fig. 1A). Combined transfection with both siRNAs clearly knocked down the target genes, in comparison with control siRNA (Fig. 1B). GL261 cells transfected with both siRNAs or either alone proliferated almost as rapidly as cells transfected with control siRNA (Fig. 1C). Transfection with either ZEB1 or ZEB2 siRNA alone moderately inhibited invasive properties in comparison with control siRNA (Fig. 1D). Of the two, ZEB2 siRNA was slightly more effective than ZEB1 siRNA (Fig. 1D). However, transfection with both siRNAs had the most potent effects. Consistent with our previous reports (6), we chose double knockdown using both siRNAs, rather than either alone, to further evaluate the roles of ZEBs in glioma cells. Allograft implantation revealed that mice inoculated with double-knockdown GL261 cells lived comparably slightly longer than those transfected with control GL261 cells, but the difference was not significant (Fig. 1E, and data not shown). These findings suggest that silencing of both ZEB1 and ZEB2 is more effective at reducing aggressive phenotypes in mouse glioma cells than silencing of either protein alone.
Figure 1.
Knockdown of ZEB1 and ZEB2 in mouse glioma cells. (A) After transfection of mouse glioma GL261 cells with control siRNA (NC) or three different kinds of siRNAs targeting mouse ZEB1 (i-iii) or ZEB2 (i-iii), expression of ZEBs was examined at the protein level by immunoblot analysis. (−) indicates no transfection. (B-D) GL261 cells without transfection (−), transfected with control siRNA (NC), or transfected with siRNAs against both ZEB1 (i) and ZEB2 (iii) or either alone were subjected to immunoblot analysis using a stripping and reblotting procedure (B), (C) MTT assay with one-way factorial ANOVA followed by Fisher's PLSD test (n.s, not significant), and (D) Boyden chamber assay with one-way factorial ANOVA followed by Fisher's PLSD test (**P<0.01). Each value represents the mean ± sd. of triplicate determinations from a representative experiment. (C and D) Similar results were obtained in at least two independent experiments. (E) At 12 h after transfection with siRNAs, BALB/cAJcl nude mice were implanted with GL261 cells pretransfected with control siRNA (NC) or both ZEB1 (i) and ZEB2 (ii) siRNAs. The mice were reared for 90 days, followed by Kaplan-Meier survival analysis (n=7).
Anti-tumor effects of ZEBs siRNAs in human glioma cells
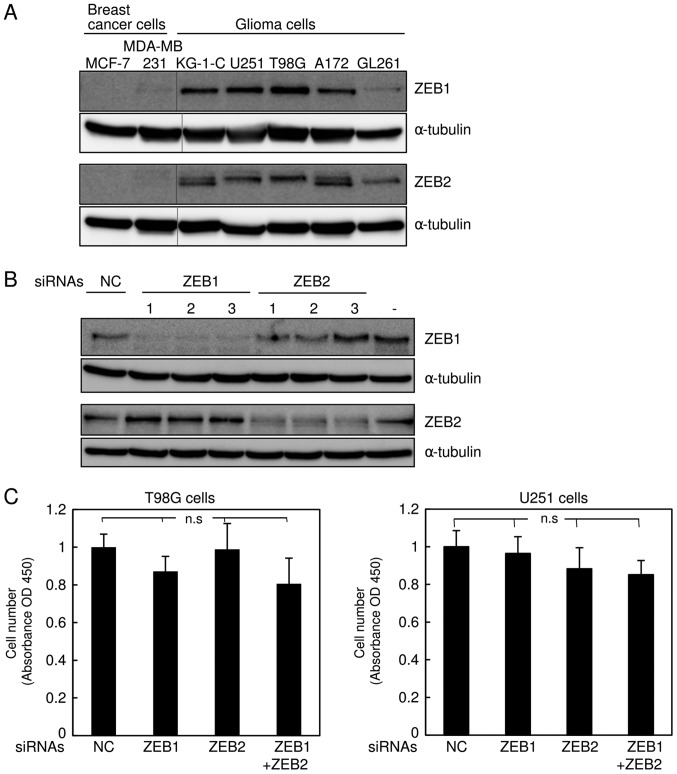
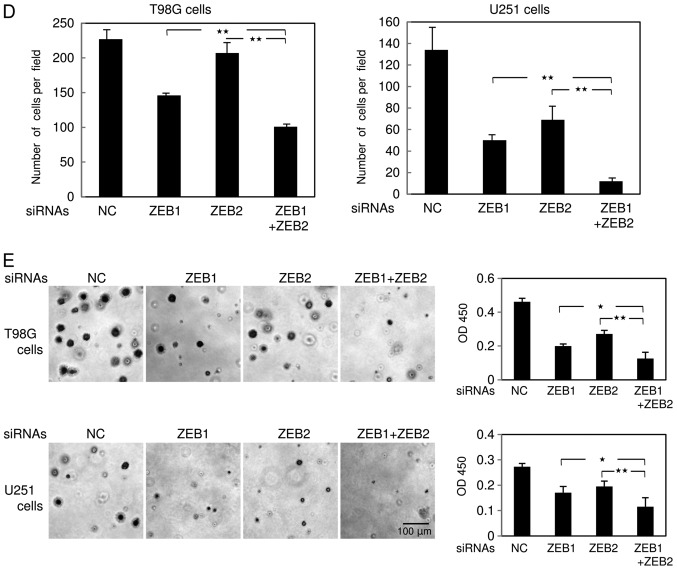
The δEF1 family proteins ZEB1 and ZEB2 are highly expressed in the basal-like subtype of breast cancer cells (6,11). Recently, we found that ZEB1 is much more highly expressed in glioma KG-1-C and U251 cells than in the basal-like breast cancer cell line MDA-MB-231 (7). Hence, we performed immunoblot analyses to examine the protein levels of ZEB2, as well as ZEB1, in several types of human glioma cells (KG-1-C, U251, T98 G, and A172) and mouse glioma cells (GL261), and compared them with two subtypes of human breast cancer cells. Consistent with our previous findings (6,12), ZEB1 and ZEB2 were negligibly expressed in the luminal breast cancer cell line MCF7, but detectable in MDA-MB-231 cells (Fig. 2A). Importantly, expression of ZEB2, as well as ZEB1, was much higher in all human glioma cells than in MDA-MB-231 cells (Fig. 2A). The level of ZEB1 in mouse GL261 cells was apparently similar to that in MDA-MB-231 cells, possibly due to the lower affinity of the ZEB1 antibody to mouse antigen, whereas ZEB2 was expressed at levels similar to those in other human glioma cells. Next, we assessed the efficacy of the siRNAs targeting human ZEB1 and ZEB2 in human glioma T98G and U251 cells (Fig. 2B and data not shown), and confirmed that these siRNAs specifically silenced their target genes. Similar to the findings shown in Fig. 1C, these siRNAs did not affect the proliferation rate of T98G or U251 cells (Fig. 2C). Cells simultaneously transfected with both ZEB1 and ZEB2 siRNAs exhibited considerable reduction of invasive properties in comparison with those transfected with control or either siRNA alone (Fig. 2D). The anti-tumor effects of both siRNAs in T98G and U251 cells were confirmed by colony formation assay in soft agar (Fig. 2E). As with the findings shown in Fig. 1, these observations suggest that silencing of both ZEBs, which is more potent than that of either alone, dramatically inhibits invasiveness of human glioma cells without affecting cell proliferation.
Figure 2.
Knockdown of ZEB1 and ZEB2 in human glioma cells. (A) Expression levels of ZEB1 and ZEB2 were examined by immunoblot analysis in MCF7 cells (the luminal subtypes), MDA-MB-231 cells (the basal-like subtypes), and several types of glioma cells. (B) After transfection of human glioma T98G cells with control siRNA (NC) or three different kinds of siRNAs targeting human ZEB1 (1–3) or ZEB2 (1–3), expression of ZEBs was examined by immunoblot analysis. (−) indicates no transfection. (C-E) After transfection of T98G and U251 cells with control siRNA (NC) or siRNAs against both ZEB1 (1) and ZEB2 (3) or either alone, the cells were subjected to the MTT assay, and (C) the results were analyzed by one-way factorial ANOVA followed by Fisher's PLSD test (n.s, not significant). (D) The results of Boyden chamber assay were analyzed by one-way factorial ANOVA followed by Fisher's PLSD test (**P<0.01). (E) After photographs were acquired (magnification, ×200), the results of the colony formation assay were analyzed by one-way factorial ANOVA followed by Fisher's PLSD test (*P<0.05, **P<0.01). Each value represents the mean ± standard deviation of triplicate determinations from a representative experiment. (C-E) Similar results were obtained in at least two independent experiments.
Expression of ZEB1 and ZEB2 in specimens from glioma patients
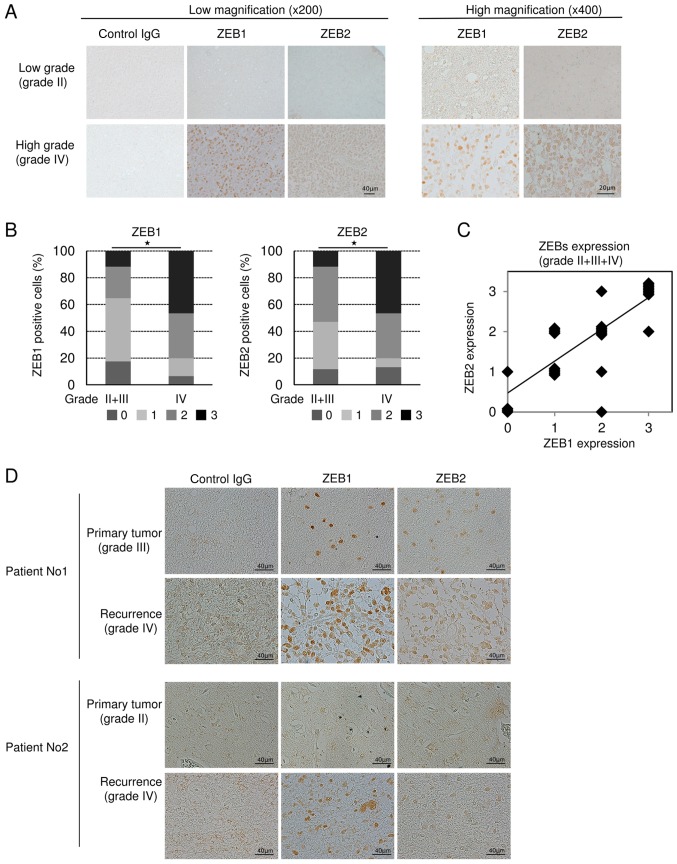
To determine whether expression of ZEBs is correlated with pathological grade or aggressiveness of glioma patients, we prepared 32 specimens from 25 patients who had been treated at our university hospital. In grade II glioma, ZEB1 and ZEB2 were marginally or negligibly expressed, but were clearly detectable in grade IV. ZEB1 and ZEB2 were localized in the nucleus and sometimes in the cytoplasm (Fig. 3A). Statistical analyses revealed that ZEB-positive cells were significantly more abundant in grade IV glioma than in grade II+III (Fig. 3B). Similarly, ZEB1-positive cells were significantly more abundant in grade IV glioma than in grade II, whereas ZEB2 was more highly, but not significantly, expressed in grade IV than in grade II (data not shown). However, the difference in ZEB expression between grades III and IV could not be statistically analyzed due to the small number of grade III specimens (n=4). A positive correlation between the levels of ZEB1 and ZEB2 was detected in grades II, III, and IV (Fig. 3C), suggesting that expression of not only ZEB1 but also ZEB2 is positively correlated with glioma grade. Importantly, some of our subjects had experienced recurrent glioma after primary surgery. One of these patients had been diagnosed initially as grade III glioma, and the other as grade II; both patients were secondarily diagnosed as grade IV glioblastoma multiforme at recurrence. IHC analyses using anti-ZEB1 and anti-ZEB2 antibodies revealed that, in both of these patients, the number of ZEB-positive cells was dramatically higher in the specimens following recurrence (Fig. 3D), suggesting that expression of ZEBs is positively correlated with aggressiveness of human glioma.
Figure 3.
Immunohistochemical analysis of ZEB1 and ZEB2 in human glioma specimens. (A) Representative images of immunohistochemical staining with control IgG, anti-ZEB1 antibody, and anti-ZEB2 antibody are shown: Grade II (n=11), grade III (n=4), and grade IV (n=15). (B) Intensity of ZEB1- and ZEB2-positive cells was scored: 0 for negative (fewer than 1% positive cells in a field); 1 for weak (1–24%); 2 for intermediate (25–49%); and 3 for strongly positive (>50%). Data were subjected to statistical analyses using non-parametric Mann-Whitney U tests (*P<0.05). (C) Correlation analysis of ZEB1 and ZEB2 expression was performed in grade II, III, and IV by Spearman's rank correlation coefficient tests [r=0.809 (P<0.01)]. (D) Initial resected tumor and recurrent tumor with malignant transformation in two patients.
Discussion
Malignant glioma exhibits aggressiveness with high infiltration properties, causing clinical therapeutic difficulties (1). Pathological grade and invasive properties are well correlated, and higher pathological grade corresponds to poorer prognosis. Consequently, chemotherapy in combination with radiation therapy is a standard postoperative treatment. In vitro and in vivo experiments have shown that tumor cells located around the tumor nest or at the invasion front are resistant to chemo- and radiotherapy and initiate invasion into adjacent tissue after radiation therapy (3). These cellular heterogeneities appear to result from a small proportion of tumor cells undergoing the EMT, or from cancer stem cells. Although the EMT in glioma has not been thoroughly assessed, it has been widely studied in other kinds of tumor cells, including breast, lung, and pancreatic cancer. Cells undergoing the EMT are extremely resistant to anti-tumor drugs in breast and pancreatic cancers (13,14). EMT-like phenotypes in breast cancer cells are largely dependent on expression of ZEB1 and ZEB2 (11). However, the molecular mechanisms by which ZEBs are induced only in cancer cells with high aggressiveness, such as the basal-like subtype of breast cancer cells, remain unclear. Recently, we found that activation of MEK-ERK1/2 pathway induces expression of ZEBs, and that the MEK1/2 inhibitor U0126 decreases ZEB expression in breast cancer cells (7,11). Although ZEBs were more strongly expressed in glioma cells than in the basal-like subtype of breast cancer cells (Fig. 2A), the levels of phospho-ERK1/2 did not dramatically differ between these two cell types (7), suggesting that activation of the ERK1/2 pathway alone is not sufficient to induce ZEBs. Thus, expression of ZEBs may be regulated by an unknown pathway or pathways that collaborate with the ERK1/2 pathway and is more highly activated in glioma cells.
TGF-β dramatically induces ZEBs in many cell types, and thus acts as a key cytokine in the EMT (5). In glioma cells, pathological grade is positively correlated with the amounts of secreted TGF-β and mesenchymal marker proteins (6). When TGF-β signals in glioma cells are ameliorated by chemical inhibitors, tumor growth and stemness properties are dramatically suppressed (15). Consistent with previous results (15), transient treatment with siRNA also reduced tumor growth in vivo (Fig. 1). Moreover, whole-transcriptome sequencing analyses have revealed that the frequency of mutations in IDH1 and TP53 (which encodes p53) is lower in primary glioblastomas, but higher in recurrent tumors (i.e., grade IV glioblastoma multiforme) (16). In esophageal cancer, mutant p53 contributes to the enrichment of cancer cells undergoing the EMT via upregulation of ZEB1 (17). In addition, epidermal growth factor receptor (EGFR) is frequently amplified and mutated in glioblastomas. Following treatment with an EGFR-blocking monoclonal antibody, patients with recurrent glioblastoma have a significantly superior progression-free survival and better overall survival. Taken together, these observations indicate that the MEK-ERK1/2 pathway activated by receptor tyrosine kinases, in cooperation with p53 and/or TGF-β, determines the aggressiveness of glioma cells by regulating expression of ZEBs.
The positive correlation between ZEB1 expression and invasiveness of glioblastoma was reported previously (18). In this study, we found that both ZEB1 and ZEB2 are highly expressed in high-grade and recurrent glioma, and that their levels are positively correlated (Fig. 3). Thus far, however, we have not detected any positive correlation between expression of ZEBs and overall survival. Therefore, we must further investigate this correlation using more patients with detailed clinical information. In addition, further analyses, especially of ZEB2, will require high-quality antibodies with adequate sensitivities for IHC; the antibodies used in this study were obtained commercially and may have been of insufficient quality for IHC analysis.
In summary, we showed that ZEB1 and ZEB2 are more highly expressed in grade IV glioma than in grades II and III. ZEB-targeted diagnosis and therapy for high grade gliomas would require development of high-quality antibodies that simultaneously recognize both ZEB1 and ZEB2, as well as anti-tumor drugs that simultaneously target both proteins. Our findings show that ZEBs are promising diagnostic and therapeutic targets for glioma and/or glioblastoma multiforme.
Acknowledgements
The authors would like to thank Dr T. Nakazawa (Department of Pathology, University of Yamanashi, Yamanashi, Japan) for the immunohistochemical analyses and valuable discussions.
Funding
This work was supported by JSPS KAKENHI (grant nos. JP15H05018 and 26462178).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
KS, TK, and KE performed experiments and analyzed data. KM, and HK drafted manuscript. TK, and MS conceived and designed the project, and drafted manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the University of Yamanashi and written informed consent was obtained from all participants. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Zaravinos A. The regulatory role of MicroRNAs in EMT and cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitoh M. Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression. Cancer Sci. 2015;106:481–488. doi: 10.1111/cas.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K, Saitoh M. TGF-β drives epithelial-mesenchymal transition through δEF1-mediated downregulation of ESRP. Oncogene. 2012;31:3190–3201. doi: 10.1038/onc.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirakihara T, Kawasaki T, Fukagawa A, Semba K, Sakai R, Miyazono K, Miyazawa K, Saitoh M. Identification of integrin α3 as a molecular marker of cells undergoing epithelial-mesenchymal transition and of cancer cells with aggressive phenotypes. Cancer Sci. 2013;104:1189–1197. doi: 10.1111/cas.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawataki T, Yamane T, Naganuma H, Rousselle P, Andurén I, Tryggvason K, Patarroyo M. Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin. Exp Cell Res. 2007;313:3819–3831. doi: 10.1016/j.yexcr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Shirakihara T, Horiguchi T, Miyazawa M, Ehata S, Shibata T, Morita I, Miyazono K, Saitoh M. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30:783–795. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brehar FM, Ciurea AV, Chivu M, Zarnescu O, Radulescu R, Dragu D. The development of xenograft glioblastoma implants in nude mice brain. J Med Life. 2008;1:275–286. [PMC free article] [PubMed] [Google Scholar]
- 11.Sinh ND, Endo K, Miyazawa K, Saitoh M. Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2, dependent on ERK1/2 activity, in breast cancer cells. Cancer Sci. 2017;108:952–960. doi: 10.1111/cas.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukagawa A, Ishii H, Miyazawa K, Saitoh M. δEF1 associates with DNMT1 and maintains DNA methylation of the E-cadherin promoter in breast cancer cells. Cancer Med. 2015;4:125–135. doi: 10.1002/cam4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Li H, Yan W, Yang P, Bao Z, Zhang C, Jiang T, You Y. Genetic and clinical characteristics of primary and secondary glioblastoma is associated with differential molecular subtype distribution. Oncotarget. 2015;6:7318–7324. doi: 10.18632/oncotarget.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB, Kalabis J, Vega ME, Kalman RA, Nakagawa M, et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70:4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.