Abstract
Lung cancer, including small-cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC), are the most common tumor types, which represent 13% of newly diagnosed cancer cases worldwide. SCLC represents 15% of all lung cancer cases. Although an increasing number of novel targeted drugs are employed for the treatment of NSCLC, including Iressa, Tarceva and Conmana, there have been almost no major breakthroughs in SCLC over the last 30 years. Therefore, new drug targets are required to treat or prevent SCLC. Aberrant Wnt signaling is associated with numerous types of tumors, and it plays a key role in cell proliferation and survival. Recent preclinical studies suggested that XAV939 is a small-molecule inhibitor of the Wnt signaling pathway. In the present study, whether XAV939 is able to inhibit the proliferation of SCLC cells and the underlying mechanism were investigated. The inhibition of cell proliferation was detected by Cell Counting Kit-8 (CCK-8) assay. The mRNA expression of β-catenin and cyclin D1 were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and the protein expression of β-catenin and cyclin D1 was determined by western blotting. The results from the CCK-8 cell viability assay confirmed that XAV939 is able to inhibit the proliferation of SCLC cells in a dose-dependent manner. However, the effects of XAV939 were not time-dependent. By contrast, the effect of DDP treatment was time- and dose-dependent. Furthermore, the effect of combination treatment with XAV939 and DDP was antagonistic at low doses and synergistic at high doses. It was also observed that the mRNA and protein expression of β-catenin and cyclin D1 was significantly in SCLC cells following XAV939 treatment compared with the control group. These findings suggested that XAV939 is able to inhibit the proliferation of H446 cells, at least partially, through downregulating the Wnt/β-catenin signaling pathway. All of these results may provide potential therapeutic approaches for the treatment of SCLC.
Keywords: small-cell lung carcinoma, WNT/β-catenin, XAV939, proliferation, cyclin D1
Introduction
Lung cancer, is the most frequently diagnosed cancer and the leading cause of cancer-associated mortalities among males and females worldwide. Small-cell lung carcinoma (SCLC) is an aggressive malignancy with a high mortality, accounting for 15% of all lung cancer cases (1). There has been a distinct lack of significant advances in SCLC therapy over the last 30 years (2). Therefore, new ways to treat or prevent SCLC are therefore needed.
In many types of cancer, Wnt signaling, which plays a fundamental role in proliferation and development, is inappropriately activated (3–5). A series of studies mentioned that Wnt signaling may be a potential therapeutic target for lung cancer (6–9). β-catenin, a key downstream effector of the signaling pathway, mediates the canonical Wnt signals. The degradation of β-catenin is regulated by glycogen synthase kinase-3, in a complex with adenomatous polyposis coli (APC) and axin. Cytosolic β-catenin levels are kept low by the destruction complex in the absence of Wnt (10–13). Effective pharmacological inhibitors of the Wnt signaling pathway have only recently been available. These inhibitors are poly-ADP-ribose polymerase (PAPR) enzymes, namely tankyrase (TNKS) 1 and TNKS2, which regulate canonical Wnt activity by promoting the stabilization of axin to increase the destruction of β-catenin (14). It has been reported that TNKS1 is upregulated in many types of cancer, including breast cancer, colon and bladder cancer as well as high-grade non-Hodgkin's lymphoma (15–19).
XAV939, a small molecule inhibitor of the dysregulated Wnt signaling pathway, is characterized as a potent inhibitor of TNKS (14). A number of recent studies have demonstrated that XAV939 is able to inhibit the growth of breast, colon and non-small cell lung cancer cells by blocking the Wnt signaling pathway (20–22). However, there rarely have been similar studies on SCLC.
The purpose of the present study was to investigate if XAV939 is able to inhibit the proliferation of SCLC cells and the underlying mechanisms. H446 cells were treated with XAV939/cisplatin (DDP) alone or combined for 24 or 48 h. The inhibition of cell proliferation was detected by Cell Counting Kit-8 (CCK-8). The mRNA and protein expression of β-catenin and cyclin D1 were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting. The results of the present study demonstrated that the anti-proliferative effects of TNKS inhibitor XAV939 may be associated with the downregulation of the Wnt/β-catenin signaling pathway in H446 cells. The present study indicated that TNKS may be a potential molecular target for the treatment of SCLC.
Materials and methods
Chemicals
XAV939 was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). DDP was purchased from Hospira Australia Pty Ltd. (Pfizer, Inc., New York, NY, USA).
Cell culture
The human H446 SCLC cells were purchased from the Institute of Biochemistry and Cell Biology (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). The cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and supplemented with 10% fetal bovine serum, penicillin G sodium (100 U/ml; all purchased from Gibco; Thermo Fisher Scientific, Inc.) and streptomycin sulfate (100 mg/ml) at 37°C under an atmosphere of 5% CO2.
CCK-8 cell viability analysis
The effect of XAV939 on the viability of H446 cells was analyzed by CCK-8. The experiment was divided into three groups: XAV939, DDP and combination group. There were 5 concentration gradients for each treatment group. H446 cells (1×105) were plated and treated in 96-well plates. The cells were treated with the corresponding drug for 24 or 48 h. Then, 10 µl CCK8 was added to each well. The absorbance was measured at 490 nm using a microplate reader (Multiskan™ GO microplate spectrophotometer; Thermo Fisher Scientific, Inc.). The experiment was performed in triplicate. The data was analyzed using the efficiency equation fa/fu=(D/Dm)m (fu, tumor cell survival rate; fa, tumor cell inhibition rate; fu=1-fa; D, drug concentration; Dm, effect concentration). Combination Index (CI) values were calculated using the D1/Dx1+D2/Dx2+αD1D2/Dx1Dx2 method. Synergism was considered when CI <1.
RT-qPCR analysis
The N-H446 cells were plated in 6-well plates and treated with XAV939 (10, 20 and 40 µM) for 24 h. Total RNA extraction was performed according to the TRIZOL manufacturer's protocol (Takara Bio, Inc., Otsu, Japan). The synthesis of cDNAs was performed by reverse transcription reactions using a PrimeScript™ RT reagent transcription kit (Takara Bio, Inc.). cDNA samples were subjected to qPCR using the SYBR Premix Ex Taq kit (Takara Bio, Inc.). PCR was performed with the following primers: β-catenin forward, 5′-CATTACAACTCTCCACAACC-3′, reverse 5′-CAGATAGCACCTTCAGCAC-3′; cyclin D1 forward, 5′-CATTGATTCAGCCTGTTTGG-3′ and reverse, 5′-GAATTCATCGGAACCGAACT-3′. GAPDH expression was used to normalize the Cq values. The expression of each gene was analyzed in triplicate. The data was analyzed using the 2−ΔΔCq method (23).
Western blot analysis
Following treatment with XAV939 (10, 20 and 40 µM) for 24 h, the H446 cells were collected and lysed using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China) and BCA Protein Assay Kit for determination of protein concentration (Beyotime Institute of Biotechnology). A total of 20 µg protein was separated on 8% SDS-PAGE and transferred to a PVDF membrane. The membranes were blocked at room temperature for 2h with 5% non-fat milk. The membranes were then incubated with the following primary antibodies: Anti-β-catenin (cat no. 6387; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-cyclin D1 (cat no. 2922; Cell Signaling Technology, Inc.) and anti-β-actin (cat no. AF0003; Beyotime Institute of Biotechnology) at a dilution of 1:1,000 at 4°C overnight. Following three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG: dilution, 1:10,000; cat no. 7074; anti-mouse: dilution, 1:10,000; cat no. 7076; Cell Signaling Technology, Inc.) for 2 h at 37°C. The blots were visualized using the ECL Plus system (Thermo Fisher Scientific, Inc.).
Statistics analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. The data were presented as the mean ± standard deviation. One-way analysis of variance and paired Student's t-test, along with the post-hoc tests least significant difference test and Dunnett's test, were used for comparison between the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of XAV939 on the proliferation of H446 cells
H446 cells were treated with different concentration (2.5–40 µM) of XAV939 for 24 or 48 h. There was a significant difference in the cell inhibition rate with the increase of drug concentration (P<0.01; Table I). However, there was no significant difference in cell inhibition rate between 24 and 48 h (P>0.05; Table I). Collectively, these results indicate that the inhibitory effect of XAV939 on the proliferation of H446 cells is dose-dependent but not time-dependent (Fig. 1 and Table I). The IC50 value for XAV939 is 21.56 µM.
Table I.
Inhibition of H446 cells by XAV939.
| Groups (µM) | 24 h | 48 h | t | P-value (Comparison between treatment durations) |
|---|---|---|---|---|
| 2.5 | 19.97±3.63 | 22.90±1.56 | −0.985 | 0.428 |
| 5.0 | 27.72±3.19 | 29.92±3.65 | −2.133 | 0.167 |
| 10.0 | 36.82±2.80 | 38.85±4.55 | −0.519 | 0.655 |
| 20.0 | 49.43±3.81 | 53.95±2.77 | −2.451 | 0.134 |
| 40.0 | 59.96±2.90 | 60.12±1.95 | −0.066 | 0.954 |
| F | 72.175 | 77.284 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
Figure 1.
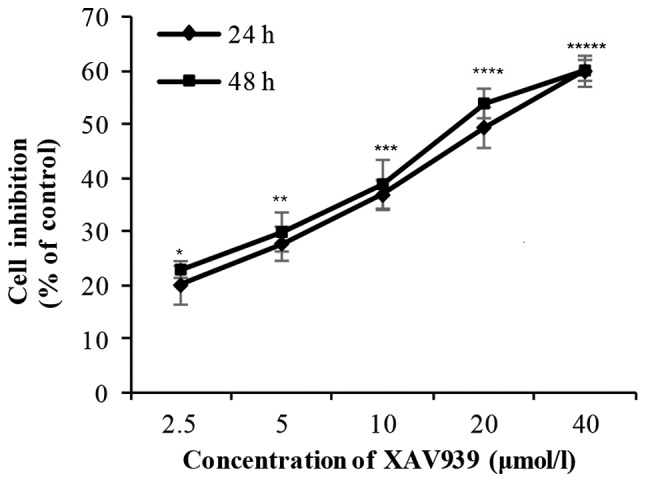
Effect of XAV939 on the viability of H446 cells that were incubated with different concentrations for different time points (24 and 48 h). Cell viability was detected by Cell-Counting-Kit 8 assay. Significant difference was observed when the drug concentration was increased (*P<0.01 vs. 2.5 µM, **P<0.01 vs. 5.0 µM. ***P<0.01 vs. 10 µM. ****P<0.01 vs. 20 µM and *****P<0.01 vs. 40 µM). However, no significant difference was found as the duration of incubation increased (P>0.05). Data are presented as the mean ± standard deviations from three independent experiments.
Effect of DDP on the proliferation of H446 cells
The cells were incubated with 0.5–8 mg/l DDP for 24 or 48 h. The cell inhibition rate increased markedly in a dose- and time-dependent manner (Fig. 2 and Table II). The IC50 value for DDP is 7.91 µM.
Figure 2.
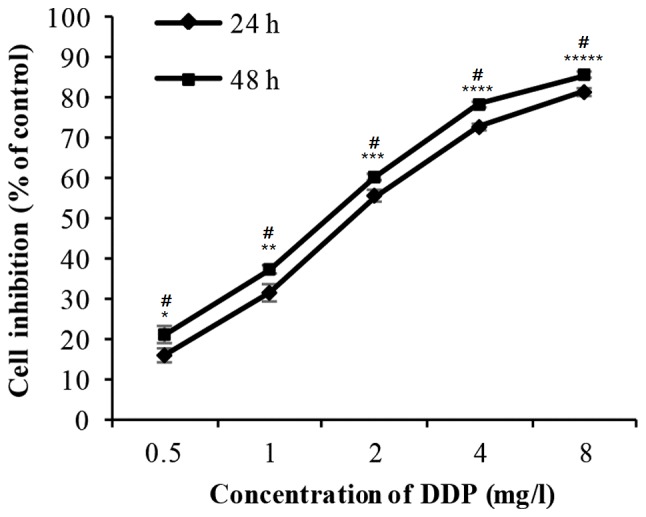
Effect of DDP on the viability of H446 cells when incubated with different concentrations for different time points (24 and 48 h). Cell viability was detected by Cell-Counting-Kit 8 assay. Significant differences were observed as the concentration of DDP increased (*P<0.01 vs. 0.5 mg/l, **P<0.01 vs. 1 mg/l, ***P<0.01 vs. 2 mg/l, ****P<0.01 vs. 4 mg/l and ****P<0.01 vs. 8 mg/l) and the duration of incubation increased (#P<0.05 vs. 24 h). Data are presented as the mean ± standard deviation from three independent experiments. DDP, cisplatin.
Table II.
Inhibition of H446 cells by cisplatin.
| Groups (mg/l) | 24 h | 48 h | t | P-value (Comparison between treatment durations) |
|---|---|---|---|---|
| 0.5 | 17.53±1.74 | 22.61±2.14 | −4.69 | 0.043 |
| 1.0 | 29.84±2.15 | 36.54±1.03 | −10.267 | 0.009 |
| 2.0 | 50.15±1.48 | 56.34±0.80 | −4.795 | 0.041 |
| 4.0 | 64.91±0.81 | 71.29±0.70 | −7.669 | 0.017 |
| 8.0 | 72.59±0.97 | 77.47±0.76 | −4.922 | 0.039 |
| F | 703.827 | 1091.974 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
Effect of XAV939 and DDP combination treatment on the proliferation of H446 cells
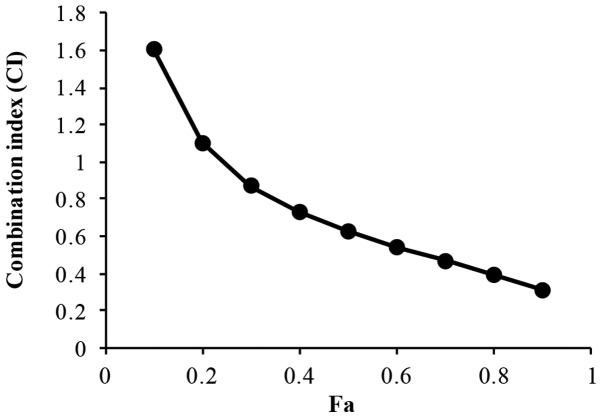
The treatment of H446 cells with a combination of XAV939 and DDP (2.5–40 µM XAV939 and 0.5–8 mg/l DDP; 1:1 ratio) for 24 or 48 h resulted in an increase in cell inhibition rate in a dose- and time-dependent manner (P<0.01; Table III). The IC50 value for XAV939 and DDP combination treatment is 7.98 µM. However, the effect of combination treatment with XAV939 and DDP was antagonistic at low doses in H446 cells (CI>1), whilst at higher concentrations the effect was synergistic (CI<1) (Fig. 3 and Table IV).
Table III.
Inhibition of H446 cells by a combination of XAV939 and DDP.
| Groups | 24 h | 48 h | t | P-value (Comparison between treatment durations) |
|---|---|---|---|---|
| 0.5 mg/l DDP + 2.5 µM XAV939 | 15.90±1.22 | 21.09±0.82 | −12.189 | 0.007 |
| 1 mg/l DDP + 5 µM XAV939 | 31.48±0.71 | 37.33±1.15 | −10.818 | 0.008 |
| 2 mg/l DDP + 10 µM XAV939 | 55.68±1.17 | 60.34±1.69 | −2.869 | 0.103 |
| 4 mg/l DDP + 20 µM XAV939 | 72.79±1.92 | 78.40±1.85 | −20.774 | 0.002 |
| 8 mg/l DDP + 40 µM XAV939 | 81.49±0.93 | 85.85±0.81 | −4.482 | 0.046 |
| F | 1437.682 | 1248.72 | – | – |
| P-value | <0.0001 | <0.0001 | – | – |
DDP, cisplatin.
Figure 3.
Graph depicting the CI plotted against the FA. (2.5–40 µM XAV939 and 0.5–8 mg/l DDP; 1:1 ratio). CI, combination index; FA, fraction affected; DDP, cisplatin.
Table IV.
Inhibition of H446 cells with single or combination treatments of XAV939 and DDP.
| Groups | Combination index | Correlation index | IC50 (µM) |
|---|---|---|---|
| XAV939 | 0.652 | 0.997 | 21.56465 |
| DDP | 0.940 | 0.965 | 7.910076 |
| XAV939 + DDP | 1.162 | 0.996 | 7.980187 |
DDP, cisplatin.
Effect of XAV939 on the mRNA expression levels of β-catenin and cyclin D1 in H446 cell
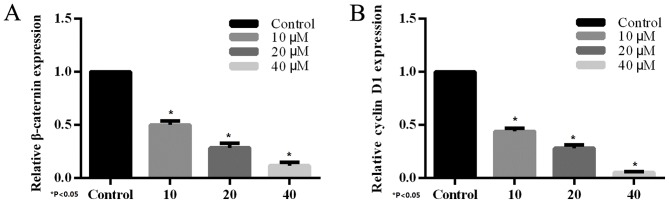
The effect of XAV939 on the expression of endogenous Wnt-regulated target genes, β-catenin and cyclin D1 was determined by RT-PCR. H446 cells were treated with various concentrations (10, 20 and 40 µM) of the tankyrase inhibitor, XAV939, for 24 h. Compared with the control group, treatment with XAV939 was able to inhibit Wnt-associated expression of target genes in H446 cells. The effects on gene expression were dose-dependent (Fig. 4) and the differences were statistically significant.
Figure 4.
Reverse transcription-quantitative polymerase chain reaction assay of (A) β-catenin and (B) cyclin D1 expression. H446 cells were treated with different concentrations of XAV939 for 24 h. The expression of β-catenin and cyclin D1 compared with the blank control. The expression of β-catenin and cyclin D1 is negatively regulated by XAV939. *P<0.05 vs. control.
Effect of XAV939 on the expression of β-catenin and cyclin D1 proteins in H446 cells
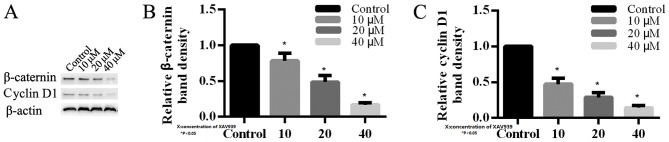
In order to investigate the mechanism of XAV939 in H446 cells, the expression levels of key proteins involved in Wnt signaling pathway were analyzed by western blotting. β-catenin was observed to be reduced in H446 cells that were treated with 10, 20 and 40 µM XAV939 compared with the control (Fig. 5). Additionally, cyclin D1 was downregulated in H446 cells following treatment with XAV939, which is an inhibitor of the Wnt signaling pathway. The effects of XAV939 on the levels of expression of β-catenin and cyclin D1 proteins were dose-dependent (Fig. 5). Altogether these results demonstrated that XAV939 may affect associated target genes in order to block Wnt transcriptional responses in SCLC cells.
Figure 5.
H446 cells were treated with different concentrations of XAV939 for 24 h. (A) Western blotting analysis of β-catenin and cyclin D1 expression in H446 cells compared with β-actin. The expression of (B) β-catenin and (C) cyclin D1 is inhibited by XAV939 compared with the blank control. Each bar represents the mean ± standard deviation from three independent experiments. *P<0.05.
Discussion
As an increasing number of targeted drugs are to be used for the treatment of non-small cell lung carcinoma (NSCLC), the overall survival and disease-free survival (DFS) of patients are markedly prolonged. On the contrary, the treatments for patients with SCLC are progressing slowly (2). Therefore, there is an urgent requirement for the identification of effective targets in order to improve patient survival.
In 1973, Sharma et al (24) identified the Wingless gene (Wg) that leads to a wingless phenotype in drosophila embryo research. Nusse et al (25) identified the Int-1 gene in mouse breast cancer in in 1982. In 1987, the study confirmed that Wg is the homologous gene of Int-1 (26), therefore Wg and Int-1 are named as Wnt genes. Aberrant WNT signaling pathway is associated with a wide array of tumor types, including colorectal cancer, acute myeloid leukemia, breast cancer, ovarian cancer and NSCLC (3,5,27,28). Therefore, Wnt signaling pathway may provide a potential therapeutic target for SCLC.
A family of secreted lipid-modified Wnt protein ligands activate the pathway in order to promote the nuclear accumulation of β-catenin by binding to a family of 7-transmembrane Frizzled (in the canonical Wnt signaling pathway (29). β-catenin forms complexes with the transcription factors T-cell factors (TCFs) and lymphoid enhancer-binding factor in the nucleus, and this reduces the expression of TCF responsive target genes, including critical growth-regulators, such as cyclin D1, and c-Myc (30,31). The β-catenin destruction complex, which consists of APC, axin, casein kinase 1 and glycogen synthase kinase-3β, downregulates the level of β-catenin (12). XAV939 is a small molecule inhibitor of the WNT signaling pathway, which is able to block WNT signaling through upregulating the destruction of β-catenin and stabilizing the axin protein.
In order to demonstrate that XAV939 is able to inhibit the growth of SCLC cells, CCK-8 assay was employed. A significant difference was observed in the rate of proliferation following treatment with XAV939. The effect of XAV939 was dose-dependent but not time-dependent.
DDP, a common chemical anti-tumor drug is still used in the clinic for the treatment of SCLC. Due to serious side effects, DDP is limited in clinical use. Therefore, there is a requirement to identify a drug that is able to achieve the therapeutic effect of the original dose of DDP that can be used in combination with a lower dosage of DDP.
Consistent with the findings of the XAV939 treatment group, a significant difference in the inhibitory rate of H446 cells following treatment with DDP was observed. However, the effect of DDP was dose-dependent and time-dependent. Following treatment with a combination of XAV939 and DDP, it was observed that the effects were antagonistic at low doses and synergistic at high doses. The drugs played their own role, and no marked synergistic effect was observed when the dose of XAV939 was low. It is possible to attain the optimum curative effect and the least adverse reactions when an appropriate dosage ratio is identified.
In order to further elucidate the mechanism of XAV939 in SCLC, Wnt-associated target genes were analyzed by RT-qPCR, and the expression of the associated proteins were examined by western blotting. In the present study, the levels of β-catenin and cyclin D1 were downregulated following the treatment of XAV939 for 24 h. All of these results suggested that XAV939 is able to downregulate β-catenin, the primary Wnt signaling effector and reduce the critical growth regulator cyclin D1.
In summary, the present study confirmed that the inhibition of XAV939 is marked in SCLC cells. Additionally, the mechanism of XAV939 may be associated with the suppression of Wnt/β-catenin signaling pathway. Further studies of the small molecule inhibitor, XAV939, in vivo are needed.
Acknowledgements
Not applicable.
Funding
Financial assistance was provided by the Affiliated Hospital of Qingdao University.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author's contributions
FP, LJY and LJZ analyzed and collected the data regarding the MTT and the western blot analysis. FZS, WXG and JXT analyzed and collected the data regarding the PCR. FP and FZS made major contributors in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Byers LA, Rudin CM. Small cell lung cancer: Where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snow GE, Kasper AC, Busch AM, Schwarz E, Ewings KE, Bee T, Spinella MJ, Dmitrovsky E, Freemantle SJ. Wnt pathway reprogramming during human embryonal carcinoma differentiation and potential for therapeutic targeting. BMC Cancer. 2009;9:383. doi: 10.1186/1471-2407-9-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, Jablons DM. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 8.Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ, Morrisey EE. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 2011;121:1935–1945. doi: 10.1172/JCI44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massagué J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 11.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 12.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rousset R, Mack JA, Wharton KA, Jr, Axelrod JD, Cadigan KM, Fish MP, Nusse R, Scott MP. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 15.Gelmini S, Poggesi M, Distante V, Bianchi S, Simi L, Luconi M, Raggi CC, Cataliotti L, Pazzagli M, Orlando C. Tankyrase, a positive regulator of telomere elongation, is over expressed in human breast cancer. Cancer Lett. 2004;216:81–87. doi: 10.1016/j.canlet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Gelmini S, Poggesi M, Pinzani P, Mannurita SC, Cianchi F, Valanzano R, Orlando C. Distribution of Tankyrase-1 mRNA expression in colon cancer and its prospective correlation with progression stage. Oncol Rep. 2006;16:1261–1266. [PubMed] [Google Scholar]
- 17.Gelmini S, Quattrone S, Malentacchi F, Villari D, Travaglini F, Giannarini G, Della Melina A, Pazzagli M, Nicita G, Selli C, Orlando C. Tankyrase-1 mRNA expression in bladder cancer and paired urine sediment: Preliminary experience. Clin Chem Lab Med. 2007;45:862–866. doi: 10.1515/CCLM.2007.133. [DOI] [PubMed] [Google Scholar]
- 18.MacNamara B, Wang W, Chen Z, Hou M, Mazur J, Gruber A, Porwit-MacDonald A. Telomerase activity in relation to pro- and anti-apoptotic protein expression in high grade non-Hodgkin's lymphomas. Haematologica. 2001;86:386–393. [PubMed] [Google Scholar]
- 19.Klapper W, Krams M, Qian W, Janssen D, Parwaresch R. Telomerase activity in B-cell non-Hodgkin lymphomas is regulated by hTERT transcription and correlated with telomere-binding protein expression but uncoupled from proliferation. Br J Cancer. 2003;89:713–719. doi: 10.1038/sj.bjc.6601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao R, Christova T, Song S, Angers S, Yan X, Attisano L. Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS One. 2012;7:e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72:2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 22.Busch AM, Johnson KC, Stan RV, Sanglikar A, Ahmed Y, Dmitrovsky E, Freemantle SJ. Evidence for tankyrases as antineoplastic targets in lung cancer. BMC Cancer. 2013;13:211. doi: 10.1186/1471-2407-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- 25.Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- 26.Siegfried E, Perrimon N. Drosophila wingless: A paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- 27.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 28.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 29.Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/MCB.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.