Figure 3.
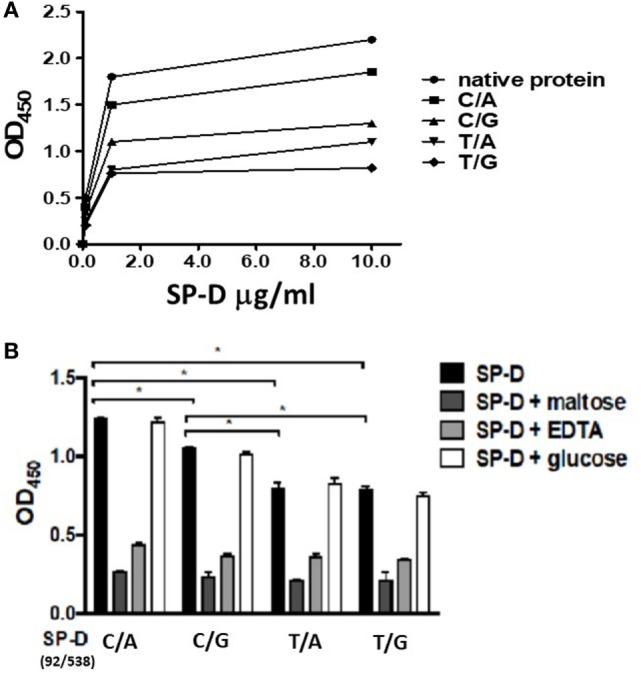
(A) Dose-binding curves of native and four genetic variants of recombinant SP-D (rSP-D) proteins to Mycobacterium bovis bacillus Calmette–Guérin (M. bovis BCG). rSP-D protein (1 and 10 µg/ml) were added to wells coated with ultraviolet-killed M. bovis BCG (106 bacteria), then quantitated using solid-phase bacterial ELISA. (B) Inhibition effects of maltose and EDTA on the binding activity of four variants of rSP-D to M. bovis BCG. Surfactant proteins D (SP-D) protein (1 µg/ml) in PBS with 2 mM CaCl2 was added into M. bovis BCG-coated wells alone, or with 10 mM EDTA, or 100 mM maltose, or 100 mM glucose. The binding activity to M. bovis BCG of rSP-D with residue 11Thr [(92C/538A) and (92C/538G)] was significantly higher than that of rSP-D with residue 11Met [(92T/538A) and (92T/538G)] (*P < 0.05). C/A: 11Thr/160Thr; C/G: 11Thr/160Ala; T/A: 11Met/Thr; T/G: 11Met/160Ala.
