Abstract
Preeclampsia (PE) is a syndrome manifested by high blood pressure that could develop in the latter half of pregnancy; however, the underlying mechanisms are not understood. Recent evidence points to the function of noncoding RNAs (ncRNAs) as novel regulators of the invasion, migration, proliferation, and apoptosis of trophoblasts involved in the development of placental vasculature. Here, we investigated the role of long intergenic ncRNA 00473 (linc00473) in PE and the associated molecular mechanisms. The expression of linc00473 was downregulated in the placenta of patients with severe PE as revealed by qRT-PCR analysis. In vitro, linc00473 knockdown in trophoblast cell lines HTR-8/SVneo, JAR, and JEG3 significantly inhibited cell proliferation and promoted apoptosis, whereas linc00473 overexpression stimulated trophoblast proliferation. The mechanistic insights were provided by RNA-seq and qRT-PCR, which revealed that linc00473 could regulate the transcription of genes relevant to cell growth, migration, and apoptosis. In particular, linc00473 inhibited the expression of tissue factor pathway inhibitor 2 (TFPI2) through binding to lysine-specific demethylase 1 (LSD1). These results indicate that linc00473 could be involved in the pathogenesis and development of PE and may be a candidate biomarker as well as therapeutic target for this disease.
Keywords: preeclampsia, linc00473, LSD1, proliferation
Introduction
Preeclampsia (PE) is a pregnancy-related syndrome developed after 20 weeks of gestation and characterized by high blood pressure and either proteinuria or pulmonary edema, liver dysfunction, thrombocytopenia, progressive renal insufficiency, and new-onset cerebral or visual disturbances.1 PE is associated with maternal multisystem organ damage, fetal growth restriction, and intrauterine distress2, 3 and is a leading cause of perinatal complications and maternal mortality worldwide.4, 5, 6 Currently, the most efficient method of PE treatment is delivery, which, however, is applied depending on the maturity of the fetus and may not be the optimal approach in certain conditions.7 Therefore, it is important to explore specific molecular mechanisms of PE to develop effective treatment as well as prevention measures for PE.
PE has multifactorial etiopathogenesis, including inflammation,8 oxidative stress,9 endothelial dysfunction,10 disturbed balance between proangiogenic and antiangiogenic factors,11 as well as inheritance12 and dietary habits.13 During the primary development of the placenta, extravillous trophoblasts (EVTs) of the fetal origin play an important role in the migration, invasion, and remodeling of the maternal spiral arteries in the myometrium and decidua.14, 15 The process of uterine spiral artery remodeling is vital for decreasing maternal blood flow resistance and increasing uteroplacental perfusion.16 Previous studies documented excessive apoptosis of EVTs in patients with PE, resulting in poor cell proliferation and impaired capacity for migration and invasion of the spiral arteries in the myometrium, which negatively affected the conversion of the maternal arteries into large-diameter vessels necessary for fetus blood supply.17 However, despite extensive research, specific molecular mechanisms underlying EVT apoptosis and their decreased migration and invasion in PE patients are still poorly understood.
Long noncoding RNAs (lncRNAs) are RNA molecules of more than 200 nucleotides in length considered to play an important role in the regulation of gene expression both at the transcriptional and posttranscriptional level.18 Research of the past decade has implicated a number of lncRNAs in aging, neurological diseases, and cancer, thus providing a new dimension in our understanding of the molecular mechanisms involved in normal physiological processes and disease. The expression of lncRNAs is correlated with such cellular processes as apoptosis,19 cell proliferation,20 migration,21 and stem cell pluripotency.22 The recent data indicate that lncRNAs may play a role in the pathogenesis of PE by regulating trophoblast invasion and apoptosis. Therefore, the identification of PE-associated lncRNAs and the related molecular mechanisms is critical for understanding PE development and improvement of treatment strategies.
Long intergenic ncRNA 00473 (linc00473) is a 1,832-bp lncRNA located on chromosome 22q12.2, which is implicated in cancer cell growth, survival, and invasion. Thus, it has been reported that linc00473 is induced in mucoepidermoid carcinoma (MEC) associated with a unique chromosomal translocation CRTC1-MAML2 and that it promoted the proliferation and survival of human MEC cells, thus serving as a biomarker for CRTC1-MAML2-positive MEC.23 linc00473 was also shown to stimulate the growth and inhibit apoptosis in cervical cancer cells both in vitro and in vivo through suppression of interleukin enhancer-binding factor 2 (ILF-2) degradation.24 Moreover, a recent study indicated that linc00473 can play an important role in establishing pregnancy by mediating decidualization of human endometrial stromal cells.25
Based on these data, we had a hypothesis that linc00473 may be involved in the pathogenesis of PE and tested it in this study by comparing the expression of linc00473 in placental tissues of women with normal pregnancies and PE and investigating the effects of linc00473 on trophoblast proliferation, apoptosis, migration, and invasion in vitro. Our findings may provide important insights into the mechanisms underlying the regulation of trophoblast function and the pathogenesis of PE.
Results
Linc00473 Is Downregulated in the Placenta of PE Patients
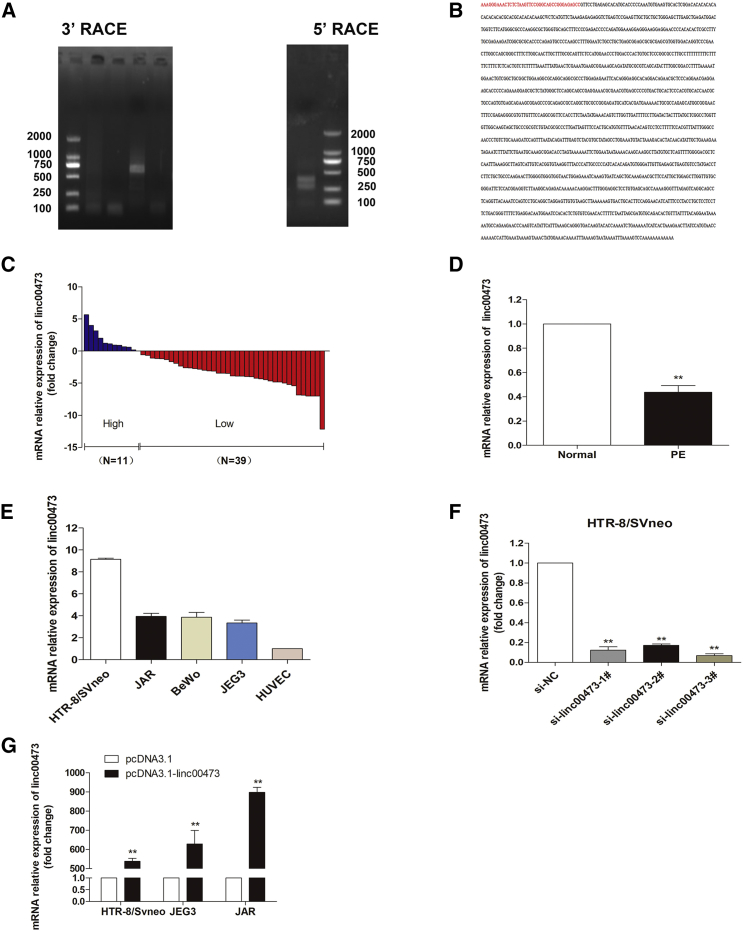
We detected and verified the full poly(A)-positive sequence of linc00473 using rapid amplification of cDNA ends (RACE) (Figures 1A and 1B). The results indicated that the full linc00473 sequence was consistent with the reference sequence (NR_026860.1) in the 3′ end; however, there was an extra 39-bp stretch (red marker) in the 5′ end compared with the reference sequence.
Figure 1.
Linc00473 Expression Is Decreased in PE
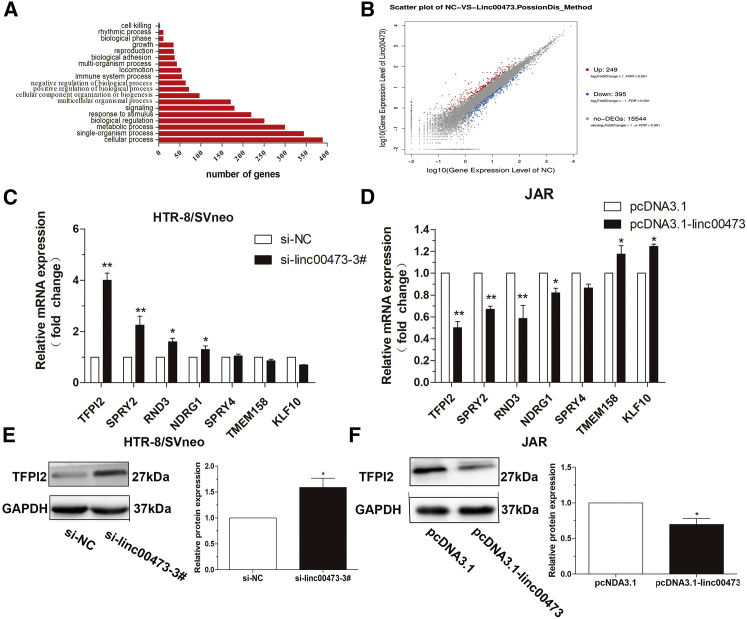
(A) Agarose gel electrophoresis of PCR products generated in 3′ (left) and 5′ (right) RACE of linc00473 in HTR-8/SVneo cells. (B) The sequence of LINC00673 at the 3′ terminus is consistent with that published in the NCBI database (NR_026860.1) but is elongated by 39 bp at the 5′ terminus (red letters). The expression of linc00473 was analyzed by qRT-PCR and normalized to that of GAPDH. (C) linc00473 levels were lower in PE placentas (n = 50) compared to normal placentas (n = 50). (D) Results are presented as the fold-change in PE placental samples relative to the control, and linc00473 expression was classified into two groups. (E) linc00473 expression in trophoblast cell lines analyzed by qRT-PCR. The levels of linc00473 in HTR-8/SVneo, BeWo, JAR, and JEG3 cells were normalized to that in HUVECs. (F and G) Relative linc00473 expression in HTR-8/SVneo, JAR, and JEG3 cells transfected with linc00473 siRNAs (F) and pcDNA3.1-linc00473 (G). The data are presented as the mean ± SD of three independent experiments; **p < 0.01.
Next, we evaluated the expression levels of linc00473 in 50 pairs of severe PE and normal placental tissues of pregnant women by qRT-PCR. Analysis of participants’ clinical characteristics (Table 1) indicated that systolic and diastolic blood pressure and proteinuria were apparently higher in PE patients than in women with normal pregnancies (p < 0.01, p < 0.01, and p < 0.05, respectively), whereas the body weight of neonates from PE mothers was lower because of early pregnancy termination. As shown in Figures 1C and 1D, linc00473 expression level in the placenta was significantly lower (p < 0.05) for 78.0% (39/50) of PE patients compared to normal pregnant women.
Table 1.
Clinical Characteristics of Patients with PE and Normal Pregnancies
| Variable | PE (n = 50) | Control (n = 50) | p Value (PE versus Control) |
|---|---|---|---|
| Maternal age (year) | 31.66 ± 4.103 | 33.28 ± 3.399 | p > 0.05 |
| Maternal weight (kg) | 74.8 ± 10.837 | 72.17 ± 9.448 | p > 0.05 |
| Smoking | 0 | 0 | p > 0.05 |
| Systolic blood pressure (mm Hg) | 162.4 ± 15.748 | 115.36 ± 7.889 | p < 0.01 |
| Diastolic blood pressure (mm Hg) | 106.6 ± 11.441 | 73.54 ± 8.581 | p < 0.01 |
| Proteinuria (g/day) | >0.3 | <0.3 | p < 0.05 |
| Body weight of infant (g) | 2,413.2 ± 1,003.853 | 3,375 ± 379.749 | p < 0.05 |
Linc00473 Influences Proliferation and Apoptosis of Trophoblasts
We then assessed linc00473 expression in four trophoblast cell lines (HTR-8/SVneo, BeWo, JEG3, and JAR) after normalization to that in other cells relevant to pregnancy (human umbilical vein endothelial cells [HUVECs]) (Figure 1E) and chose HTR-8/SVneo, JAR, and JEG3 cells to investigate linc00473 functional activity. To assess linc00473 effects in trophoblasts, linc00473 was overexpressed in the three cell lines and knocked down in HTR/SVneo cells, because linc00473 levels in JAR and JEG3 cells were relatively low and the cells were more suitable for linc00473 overexpression. qRT-PCR analysis revealed that linc00473 expression was silenced in HTR/SVneo cells more efficiently by si-linc00473-1# and si-linc00473-3# than by si-linc00473-2# (Figure 1F); therefore, si-linc00473-1# and/or si-linc00473-3# were used for subsequent experiments. The upregulation of linc00473 expression after pcDNA-linc00473 transfection was confirmed in all three cell lines (Figure 1G).
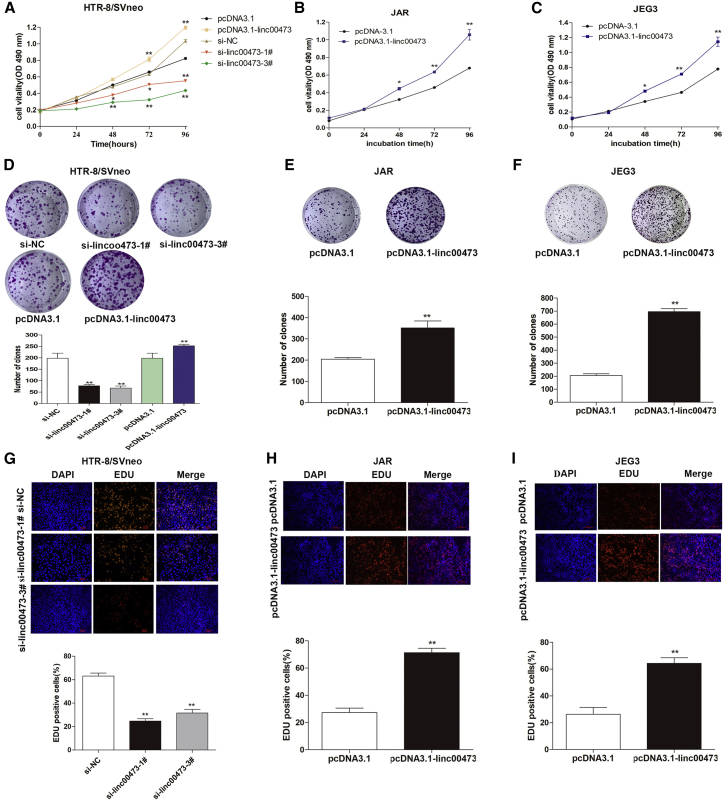
Knockdown of linc00473 expression by si-linc00473 significantly suppressed cell proliferation in trophoblast cells as evidenced by the MTT assay, whereas linc00473 overexpression promote cell proliferation (Figures 2A–2C). Knockdown of linc00473 expression by si-linc00473 dramatically diminished clonogenic survival in HTR-8/SVneo cells as indicated by the colony-formation assay (Figure 2D). At the same time, linc00473 overexpression significantly increased clonogenic survival of the cultured trophoblasts (Figures 2D–2F). The results of ethynyl deoxyuridine (EDU) immunostaining confirmed these findings (Figures 2G–2I). Overall, these data indicate that linc00473 positively regulates the proliferation of HTR/SVneo, JAR, and JEG3 trophoblast cells, suggesting that linc00473 deficiency can contribute to the development of PE.
Figure 2.
Linc00473 Promotes Trophoblast Proliferation In Vitro
(A–C) The viability of si-linc00473-transfected HTR-8/SVneo cells (A) or pCDNA3.1-linc00473-transfected JAR (B) and JEG3 (C) cells were analyzed by the MTT assay. (D–F) The proliferation of si-linc00473-transfected HTR-8/SVneo cells (D) and pcDNA3.1-linc00473-transfected HTR-8/SVneo, JAR (E), and JEG3 (F) cells was evaluated by the colony formation assay, and the number of colonies was counted. (G–I) Proliferating HTR-8/SVneo (G), JAR (H) and JEG3 (I) cells are marked with EDU (red) and cell nuclei are stained with DAPI (blue). The data are presented as the mean ± SD of three independent experiments; *p < 0.05 and **p < 0.01.
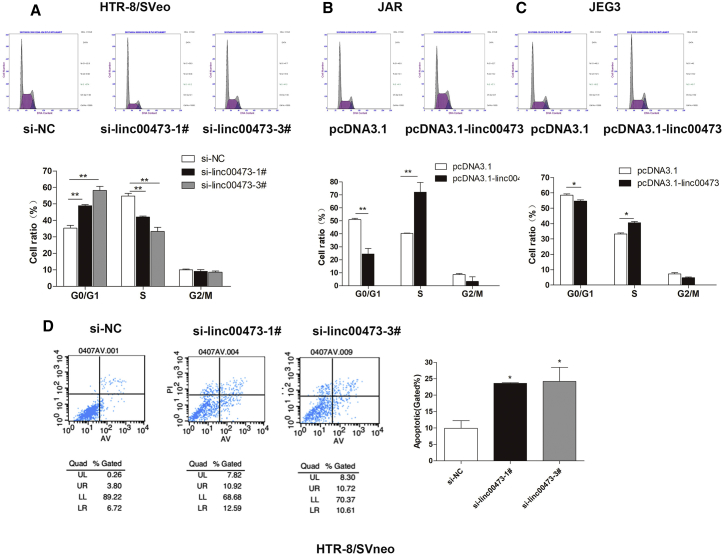
Furthermore, we found that linc00473 was involved in the regulation of cell cycle progression and apoptosis in trophoblasts. Flow cytometry analysis revealed that linc00473 knockdown resulted in cell cycle arrest at the G0/G1 phase, whereas linc00473 overexpression reversed the effect (Figures 3A–3C). Consistent with these results, the apoptosis rate in HTR/SVneo trophoblasts increased after linc00473 knockdown by specific siRNAs (Figure 3D).
Figure 3.
Effects of linc00473 on Trophoblast Cell Cycle and Apoptosis In Vitro
HTR-8/SVneo trophoblasts were transfected with linc00473-specific siRNAs (si-linc00473-1# and si- linc00473-3#) and JAR and JEG3 trophoblasts were transfected with pcDNA3.1-linc00473. (A–C) Cell cycle was analyzed by flow cytometry in HTR-8/SVneo (A), JAR (B) and JEG3 (C) cells. Representative FACS images and related statistics are shown. (D) Cell apoptosis rates were analyzed by flow cytometry. LR, early apoptotic cells; UR, terminal apoptotic cells. The data are presented as the mean ± SD of three independent experiments; *p < 0.05 and **p < 0.01.
Linc00473 Promotes the Migration and Invasion of Trophoblasts In Vitro
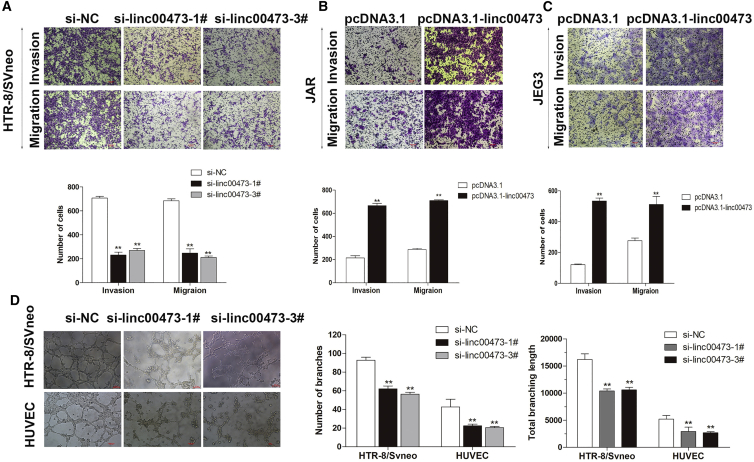
There is little doubt cell metastasis is an important part of cancer progression and may worsen the outcomes of patients with trophoblast migration and invasion, which are important for the establishment of the blood flow between the mother and embryo, and the impairment of these processes can result in PE. Therefore, we evaluated the effects of linc00473 on the migration and invasion abilities of HTR-8/SVneo, JAR and JEG3 cells by Transwell assays. The results indicated that a decrease in linc00473 expression inhibited trophoblast migration and invasion compared to control cells, whereas linc00473 overexpression promoted them (Figures 4A–4C), suggesting that linc00473 can influence the migration and invasion of trophoblasts, which are critical for the development of placental vasculature impaired in PE patients.
Figure 4.
Effect of linc00473 on Trophoblast Migration, Invasion, and Vascularization In Vitro
(A–C) The migration and invasion capacity of cultured trophoblasts transfected with si-linc00473 in HTR-8/SVneo cells (A) or pcDNA3.1-linc00473 in JAR (B) and JEG3 (C) cells were analyzed by the Transwell assays and were significantly lower or higher, respectively, than that of control cells. (D) The number of branch points and total branching length of the network were decreased in si-linc00473-transfected HTR-8/SVneo cells and HUVECs compared to control cells. The data are presented as the mean ± SD of three independent experiments; **p < 0.01.
Linc00473 Affects Network Formation Ability of Trophoblasts In Vitro
It was reported that impaired spiral artery remodeling is associated with the pathogenesis of PE as it leads to hypoxia, placental ischemia, and the release of placental factors into the maternal blood circulation, resulting in the systemic inflammatory response and vascular endothelial injury. Therefore, we explored the role of linc00473 on the network formation ability in two types of pregnancy-related cells, HUVECs and HTR-8/SVneo trophoblasts. The knockdown of linc00473 weakened the network formation ability of both HTR-8/SVneo cells and HUVECs, as evidenced by a decrease in the number of branches and total branching length of the network compared to control cells (Figure 4D).
Linc00473 Influences Gene Expression in Trophoblasts
We next performed RNA transcriptome sequencing in scrambled negative control siRNA (si-NC) and si-linc00473-transfected HTR/SVneo cells to explore linc00473-associated changes in gene expression. A total of 249 and 395 transcripts were increased or decreased, respectively, in cells with linc00473 deficiency (Figure 5B; Table S2). Evaluation of the biological pathways activated by linc00473 using the gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases indicated that genes related to cell growth were deregulated in linc00473 knockdown cells (Figure 5A). Expression changes in HTR-8/SVneo and JAR cells were further confirmed by qRT-PCR. Among the transcripts related to cell growth, apoptosis, and migration, the expression of tissue factor pathway inhibitor 2 (TFPI2) was significantly upregulated after linc00473 knockdown in HTR-8/SVneo cells (Figure 5C) and downregulated by linc00473 overexpression in JAR cells (Figure 5D). Consistent with these results, western blotting analysis indicated similar effects of linc00473 on TFPI2 protein expression in HTR-8/SVneo and JAR cells (Figures 5E and 5F).
Figure 5.
Linc00473 Knockdown Affected the Expression of Genes Involved in Cell Proliferation and Migration
(A and B) Gene expression profiling was performed by RNA-seq in HTR-8/SVneo cells transfected with si-linc00473. Differentially expressed genes are shown (A). GO analysis of genes differentially expressed in si-NC- and si-linc00473-transfected cells. Cell growth was among significant biological processes affected by linc00473 depletion in trophoblasts as evidenced by the number of genes with altered expression (B). (C and D) Relative mRNA expression of genes involved in cell proliferation and migration in linc00473-deficient HTR-8/SVneo cells (C) and linc00473-overexpressing JAR cells (D) was analyzed by qRT-PCR. (E and F) TFPI2 protein expression in linc00473-deficient HTR-8/SVneo cells (E) and linc00473-overexpressing JAR cells (F) was assessed by western blotting. The data are presented as the mean ± SD of three independent experiments; *p < 0.05 and **p < 0.01.
Linc00473 Silences TFPI2 Epigenetically by Binding to LSD1
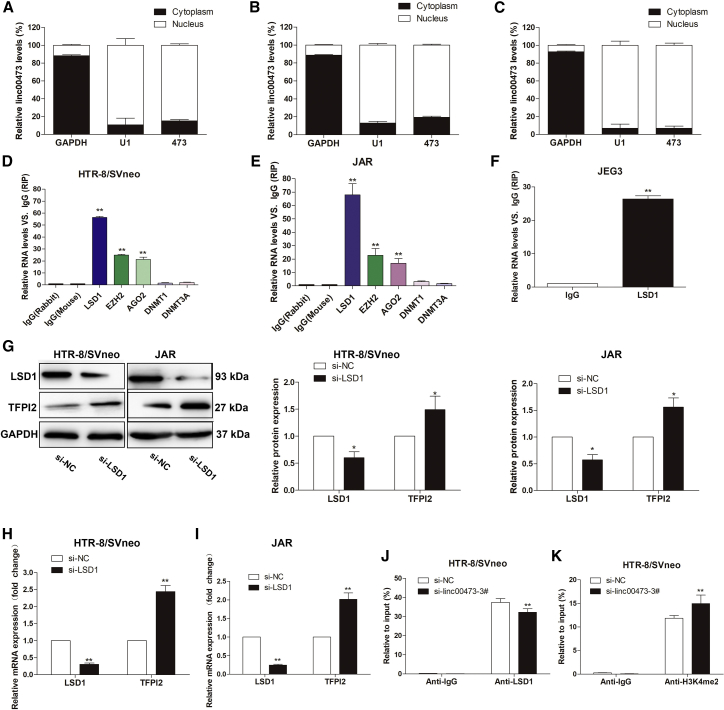
To test linc00473 effects on gene expression, we first investigated the subcellular localization of linc00473 in HTR/SVneo, JAR, and JEG3 cells using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and small nuclear RNA U1 (RNU1) as cytoplasmic and nuclear markers, respectively (Figures 6A–6C). The results indicate that linc00473 was mainly localized in the cell nucleus, supporting its role in transcriptional regulation.
Figure 6.
Linc00473 Binding to LSD1 Inhibits TFPI2 Expression
(A–C) Cell fractionation assay revealed that linc00473 was predominantly localized in the nucleus of HTR-8/SVneo (A), JAR (B), and JEG3 (C) cells. GAPDH and small nuclear RNA U1 were used as cytoplasmic and nuclear markers, respectively. (D–F) RIP assays showed that linc00473 was mainly bound to LSD1 in HTR-8/SVneo cells (D), JAR (E), and JEG3 (F) cells, respectively. (G–I) LSD1 silencing changed the expression of LSD1 and TFPI2 at the protein (G) and mRNA levels in HTR-8/SVneo (H) and JAR (I) cells. (J and K) Enrichment of LSD1 (J) and H3K4me2 (K) in the promoter region of TFPI2 detected by the ChIP assay. The data are presented as the mean ± SD of three independent experiments; *p < 0.05 and **p < 0.01.
Lysine-specific demethylase 1 (LSD1) is a flavin adenine dinucleotide (FAD)-dependent amine oxidase that specifically performs demethylation of H3K4me2 and H3K9me2,26, 27 thus affecting gene transcription. Recently, it was shown that by regulating target gene expression, LSD1 can influence tumorigenesis,28, 29 embryonic differentiation,30, 31 and the formation of euchromatin.32 Therefore, we hypothesized that linc00473 may control TFPI2 expression by recruiting LSD1 in trophoblasts. Indeed, the RNA immunoprecipitation (RIP) assay showed that linc00473 was precipitated with anti-LSD1 antibodies in HTR-8/SVneo, JAR, and JEG3 cells (Figures 6D–6F), thus confirming our hypothesis.
We then investigated the potential functional relationship between linc00473 and LSD1. The knockdown of LSD1 by a specific siRNA resulted in TFPI2 upregulation both at the protein and mRNA levels (Figures 6G–6I), suggesting that linc00473 may regulate TFPI2 expression in trophoblasts through epigenetic mechanisms. The chromatin immunoprecipitation (ChIP) assay with antibodies against LSD1 and H3K4me2 revealed the enrichment of LSD1 and H3K4me2 in the promoter region of the TFPI2 gene, which was significantly decreased for LSD1 but increased for H3K4me2 after transfection with si-linc00473 (Figures 6J and 6K).
TFPI2 Upregulated in PE Placental Tissues Influences Trophoblast Viability In Vitro
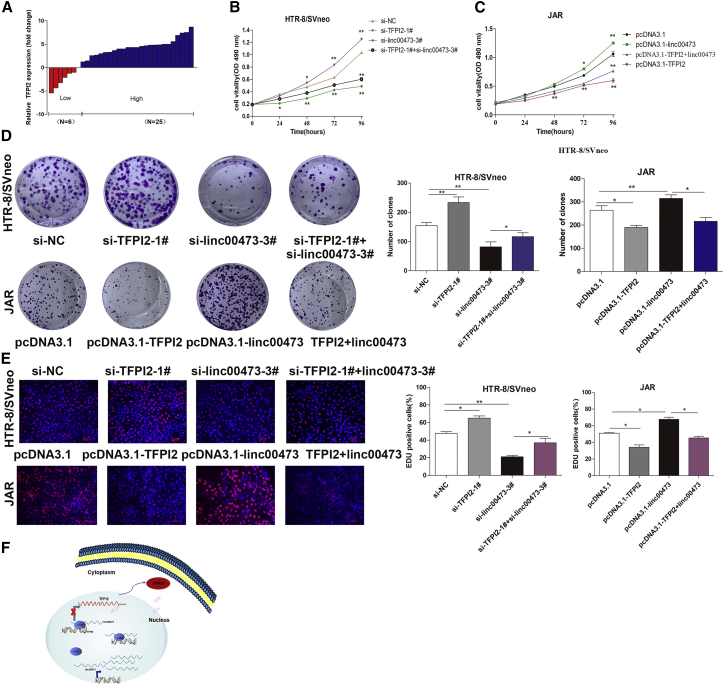
Evaluation of TFPI2 mRNA expression in 31 placental tissues of PE patients indicated that TFPI2 transcription was increased in PE compared to normal pregnancy (Figure 7A), suggesting a TFPI2 role in PE pathogenesis. In HTR-8/SVneo and JAR trophoblasts, TFPI2 overexpression significantly inhibited, whereas TFPI2 knockdown promoted = cell proliferation (Figures 7B and 7C). Finally, we examined the biological effects of TFPI2 association with linc00473 by co-transfecting HTR-8/SVneo and JAR cells with linc00473 and TFPI2 siRNAs and analyzing cell proliferation using colony-formation and EDU assays. The results indicated that si-TFPI2 could moderately rescue si-linc00473-inhibited proliferation of HTR-8/SVneo and JAR cells, and similar results were obtained by co-transfecting trophoblasts with pcDNA3.1-TFPI2 and pcDNA-linc00473 (Figures 7D and 7E).
Figure 7.
TFPI2 Inhibits Trophoblast Proliferation and Counterbalance the Activity of Linc00473
(A) Relative expression of TFPI2 (fold change) in placental tissues of PE patients compared to those of healthy pregnant women (n = 31). (B and C) MTT analysis of cell viability for si-TFPI2- (B) or pcDNA3.1-TFPI2-transfected (C) trophoblasts. (D and E) Proliferation of HTR-8/SVneo and JAR cells analyzed by the colony-formation (D) and EDU (E) assays. The data are presented as the mean ± SD of three independent experiments; *p < 0.05 and **p < 0.01. (F) A schematic pathway illustrating the role of linc00473 in the proliferation and migration of trophoblasts in PE.
Discussion
The human genome is estimated to contain approximately 16,000 non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and lncRNAs (>200 nucleotides). The lncRNAs are localized in the nucleus and are enriched in the chromatin or specific subnuclear compartments, where they can act as structural scaffolds of nuclear domains and influence chromatin organization,33 as well as transcriptional and posttranscriptional regulation of gene expression.34, 35 In our previous studies, we have shown the involvement of several lncRNAs (MEG3, TUG1, PVT1, and MVIH)36, 37, 38, 39 in the occurrence and development of PE. Thus, exploration and identification of PE-associated lncRNAs and their functions may provide a new angle in the understanding of PE pathogenesis and define novel therapeutic targets and diagnostic and prognostic markers for PE.
In recent years, it has remained the focus of research to search for the pathogenesis of PE in placental trophoblastic cells about relating biological indicators. Currently, the methods of studying the mechanism of disease includes in vivo cell study, in vitro cell culture, animal models, etc. Among them, the study of cell culture in vitro only needs a simplified cell growth environment. It is convenient to obtain a uniform cell group and to facilitate the application of experimental intervention factors. We can observe the experimental results easily in a short time, and the cells have become a common method used by many researchers. At present, more cells are studied. There are HTR-8/SVneo,40 JEG-3,41 BeWo,42 JAR,43 dNK,44 villous 3A,45 and so on. So, in our study, we explored the potential molecular mechanism of linc00473 mainly based on several different trophoblastic cell lines.
Linc00473 has been mostly investigated for its role in cancer.46, 47 In this study, we showed that linc00473 expression was dramatically decreased in the placental tissues of women with PE compared to that in women with normal pregnancies. In vitro, silencing of linc00473 expression suppressed the proliferation, cell cycle progression, invasion, and migration while promoting apoptosis of cultured trophoblasts, whereas linc00473 overexpression caused the opposite effects. Together, these findings characterize linc00473 as an important regulatory molecule involved in the control of the biological activity of trophoblasts, the main players in the development of PE, and suggest that linc00473 may be a promising biomarker for PE prediction.
According to our results, linc00473 was predominantly localized in the nucleus, indicating its possible involvement in transcriptional regulation. Indeed, we found that linc00473 could bind LSD1, suggesting that it may affect LSD1-mediated epigenetic regulatory mechanisms and, through them, influence the onset and progression of PE.
RNA sequencing (RNA-seq) and qRT-PCR analyses indicated that TFPI2 was markedly upregulated by linc00473 knockdown. TFPI2 is a matrix-associated Kunitz-type serine protease inhibitor that controls plasmin- and trypsin-mediated activation of zymogen matrix metalloproteinases involved in tumor progression and metastasis, and it was shown that TFPI2 expression inversely correlated with cancer cell invasion and migration.48, 49, 50 Consistent with these findings, TFPI2 knockdown promoted proliferation of cultured trophoblasts and counterbalanced the inhibitory effects of linc00473 deficiency. Our results also indicated that TFPI2 expression was silenced by LSD1 through epigenetic mechanisms. Based on these findings, we propose that linc00473 can inhibit TFPI2 expression by binding to LSD1 in trophoblasts, thus promoting their invasion and migration, the critical processes for proper uterine spiral artery remodeling in pregnancy, which are deregulated in PE.
In summary, our study shows that linc00473 is downregulated in placental tissues from PE patients compared with normal pregnant women and that low expression of this lncRNA may potentially serve as a prognostic biomarker of PE. Moreover, our results strongly support the role of linc00473 as a scaffold and a member of the LSD1-mediated epigenetic regulatory pathway involved in the inhibition TFPI2 expression during pregnancy. Together, the present findings suggest that linc00473 can be a novel molecular target for early diagnosis and treatment of PE (Figure 7F). Further studies are needed to elucidate other potential mechanisms through which linc00473 participates in the biological functions of trophoblasts in the context of PE.
Materials and Methods
Patients and Collection of Tissue Samples
We obtained 50 paired placental samples from women with normal pregnancies and PE patients, who underwent cesarean deliveries in Jiangsu Province Hospital from August, 2016 to December, 2017. The placenta tissue samples (about 1 cm × 1 cm × 1 cm in size) were taken from the central area of the placenta maternal surface to avoid necrosis and calcification and were immediately frozen in liquid nitrogen and subsequently used for RNA and protein extraction. Clinicopathological characteristics of the participants are summarized in Table 1. This research was authorized by the Ethnics Board of the First Affiliated Hospital of Nanjing Medical University, China, and all patients provided written informed consent.
Cell Culture
Four human trophoblast cell lines (HTR/SVneo, JAR, JEG3, and BeVo) and HUVECs were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). HTR/SVneo and JAR cells were cultured in RPMI1640, JEG3 cells in MEM, BeVo cells in F12K, and HUVECs in endothelial cell medium (ECM) (all media from KeyGEN, Nanjing, China) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen). All cells were maintained in a humidified atmosphere with 5% CO2 at 37°C.
RACE
Both 5′ and 3′ RACE were conducted using the First Choice-RLM RACE kit (Life Technologies, Carlsbad, CA, USA) following the manufacturers’ instructions.
Cell Transfection
Plasmid vectors (pcDNA, pcDNA3.1-linc00473, and pcDNA3.1-TFPI2) were purified using the DNA Midiprep kit (QIAGEN, Hilden, Germany). Three different linc00473-specific siRNAs, LSD1 and TFPI2 siRNAs, and si-NC were purchased from Invitrogen; the sequences are shown in Table S1. HTR/SVneo, JAR, and JEG3 cells were cultured in 6-well plates until 80% confluence and then transfected with siRNAs using Lipofectamine 2000 (Invitrogen, USA) or with plasmid vectors (4 g) using X-tremeGENE HP DNA Transfection Reagent (Roche, Pennsburg, Germany) following the manufacturers’ instructions. Cells were harvested 48 hr posttransfection and analyzed by qRT-PCR and western blotting.
RNA Preparation and qRT-PCR
Total RNA was isolated from tissues or cultured cells with TRizol reagent (Invitrogen), and its quality and quantity assessed using NanoDrop2000c (Thermo Fisher Scientific, Waltham, MA, USA). RNA (1 μg) was reverse-transcribed to cDNA using Primer Script RT Master Mix (Takara, Dalian, China), and qRT-PCR was performed with SYBR Premix Ex Taq (Takara, Dalian, China) and specific primers (Table S1) in an ABI 7500 system following the manufacturer’s protocol. The expression level of linc00473 was calculated by the 2−ΔΔCT method, normalized to that of GAPDH, and converted to fold changes.
Subcellular Fractionation and RNA Isolation
Cytoplasmic and nuclear RNA was isolated and purified using the PARIS Kit (Life Technologies) according to the manufacturers’ instructions.
Cell Proliferation and Colony Formation Assays
For cell proliferation, HTR/SVneo, JAR, and JEG3 cells were seeded in 96-well plates (3,000–4,000 cells/well) and transfected with si-linc00473 or pcDNA-linc00473; five replicates for each point were used. Cell viability was assessed every 24 hr using the Cell Proliferation Reagent Kit I (MTT) (Roche) based on the absorbance at 490 nm measured in an ELx-800 University Microplate Reader (Biotech, Winooski, VT, USA).
For the colony formation assay, cells were seeded in 6-well plates (800 cells/well), transfected with specific siRNA or plasmids, and cultured for 10–12 days with a change of medium at day 5. Cells were fixed with methanol and stained with 0.1% crystal violet (Sigma-Aldrich); colonies were observed under a microscope and counted using the Photoshop software. At least three independent experiments were performed.
EDU Assay
The EDU assay was performed for accurate estimation of cell proliferation as a complementary method using the 5-ethynyl-2-deoxyuridine labeling/detection kit (Ribobio, Guangzhou, China). HTR/SVneo, JAR, and JEG3 cells were seeded in 24-well plates at 2∼3 × 104 cells per well and transfected with si-linc00473-1#, si-linc00473-3#, si-NC, pcDNA-linc00473, or pcDNA for 24 hr. Then 200 mL/well of EDU labeling medium was added and cells were cultured for another 2 hr, fixed in 4% paraformaldehyde (pH 7.4) for 30 min, permeabilized with 0.5% Triton X-100 for 20 min at room temperature, washed in PBS (pH 7.4), and stained with anti-EDU solution for 30 min. After counterstaining with 250 mL DAPI (Invitrogen, Molecular Probes, Eugene, OR, USA) for 25 min, images of EDU-positive cells were captured under a fluorescence microscope (Nikon Corporation, Tokyo, Japan). The experiment was independently repeated three times.
Flow Cytometry Analysis of Cell Cycle and Apoptosis
Cells transfected with si-linc00473 or pcDNA-linc00473 were harvested 48 hr posttransfection by trypsinization, washed twice with PBS, stained with propidium iodide (PI) using the Cycle TEST PLUS DNA Reagent Kit (BD Biosciences, San Jose, CA, USA), and analyzed in a FACScan flow cytometer (BD Biosciences) equipped with a Cell Quest software (BD Biosciences) to estimate the percentage of cells in G0–G1, S, and G2–M phases. All samples were assayed in triplicate. Apoptosis was assessed by flow cytometry after double staining of the transfected cells with fluorescein isothiocyanate (FITC)-Annexin V and PI using the FITC-Annexin V Apoptosis Detection Kit (BD Biosciences), and the percentages of viable, dead, and early and late apoptotic cells were calculated and compared with those in controls.
Cell Migration and Invasion Assays
Cell migration and invasion assays were performed using 24-well Transwell chambers with 8-μm pore size polycarbonate membranes (Corning, Corning, NY, USA). Cells were plated on the top side of the membrane pre-coated or not with Matrigel (BD, Franklin Lakes, NJ, USA) for invasion or migration assays, respectively. After incubation for 36∼48 hr, cells inside the upper chamber were removed with cottons swabs, whereas those on the lower membrane surface were fixed by methanol and stained with 0.5% crystal violet solution. Five randomly selected fields were analyzed for each well.
Western Blotting Assay
HTR/SVneo and JAR cells cultured in 6-well plates were transfected with si-linc00473 or pcDNA-linc00473 and total cellular proteins were extracted with radioimmunoprecipitation assay (RIPA) protein extraction reagent (Beyotime, Beijing, China) supplemented with protease inhibitor cocktail (Roche, Pleasanton, CA, USA) and phenylmethylsulfonyl fluoride (Roche). Proteins were separated by SDS-PAGE in 10% gels and transferred to 0.22-μm polyvinylidene difluoride membranes (Sigma), which were incubated with antibodies against LSD1 (1:1,000; CST, Danvers, MA, USA) or TFPI2 (1:1,000; Proteintech); anti-GAPDH antibody (1:3,000; Santa Cruz, CA, USA) was used as control. The intensity of protein bands was quantified by densitometry using the Quantity One software (Bio-Rad). At least three independent experiments were performed.
RNA-Seq Analysis
The RNA-seq experiments were conducted in the Huada Genomics Institute (Wuhan, China), and the mRNA-seq library was obtained according to standard protocols (Illumina, San Diego, CA, USA). In brief, total RNA from si-NC- or si-linc00473-3#-transfected HTR-8/SVneo cells was isolated as described above and mRNA was purified using Dynabeads Oligo (dT) (Invitrogen Dynal) and reverse-transcribed into cDNA, which was then fragmented by nebulization to establish the mRNA-seq library. Then, the differentially expressed genes were screened by using the RSEM software and the differential gene expression patterns were analyzed by cluster analysis. GO annotation analysis of the distribution of gene functions was performed by using WEGO software (http://wego.genomics.org.cn). Also, pathway enrichment analysis of the interaction between genes was analyzed by the KEGG database.
RIP Assay
RIP assays were performed using the EZ-Magna RIP kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. HTR-8/SVneo, JAR, and JEG3 cells were grown in 15-cm plates, collected by centrifugation, and lysed in RIP lysis buffer so that 100 μL contained the lysate of 1∼2.0 × 107 cells. Cell lysates were used for immunoprecipitation with anti-LSD1, -EZH2, -AGO2, -DNMT1 and -DNMT3A antibodies and normal immunoglobulin G (IgG) (Millipore, Billerica, MA, USA) and the immunoprecipitated RNA was amplified by qRT-PCR using the Primer Script RT Master Mix kit and primers specific for linc00473 and IgG (Table S1).
ChIP Assay
ChIP assays were performed using the EZ-Magna ChIP A kit (Millipore) according to the manufacturer’s protocol. HTR-8/SVneo cells were cross-linked by 1% formaldehyde for 10 min at 25°C, lysed, and sonicated to obtain 200- to 500-bp DNA fragments. Primary LSD1 and H3K4me2-specific antibodies (Millipore) or control IgG were added to pre-cleared supernatants and the mixtures were incubated with rotation for 3 hr or overnight at 4°C. Then, Dynabeads Protein A/G were added to the mixtures and the samples were incubated with vortexing for 2 hr at 4°C. The beads were then washed sequentially with low-salt and high-salt RIPA, LiCi, and TE and DNA was isolated by phenol/chloroform extraction. ChIP-qPCR was performed using SYBR Premix Ex Taq and specific primers (Table S1), and the data were normalized to the input. At least three independent experiments were conducted.
Statistical Analysis
The statistical analyses were performed with the SPSS 17.0 statistical software (IBM, Chicago, IL, USA). Each experiment was independently repeated at least three times and the data were expressed as the mean ± SD. Student’s t test or Mann-Whitney test were used for comparisons between two groups and ANOVA or Kreskas-Wallis tests were applied for multiple comparisons. p values less than 0.05 indicated statistical significance.
Author Contributions
L.S., D.W. and Y.Z. conceived and designed the study. D.W. and Y.X. conducted the experiments and wrote the article. D.W., S.H., T.W. and S.W. collected clinical tissues and data. Q.Z., X.L., X.H. and J.W. coordinated and analyzed the data. All the authors contributed to, read and approved the final manuscript.
Conflicts of Interest
No conflicts of interest were stated.
Acknowledgments
The present work was supported by the National Scientific Foundation of China (no. 81771603). This work was partly supported by the Natural Science Foundation of Jiangsu Province (project number BK20171502) and Key Disciplines of 13th Fifth-Year Strong and Healthy Engineering in Jiangsu Province.
Footnotes
Supplemental Information includes two tables and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.05.020.
Supplemental Information
References
- 1.López-Jaramillo P., Casas J.P., Serrano N. Preeclampsia: from epidemiological observations to molecular mechanisms. Braz. J. Med. Biol. Res. 2001;34:1227–1235. doi: 10.1590/s0100-879x2001001000001. [DOI] [PubMed] [Google Scholar]
- 2.Cornelius D.C. Preeclampsia: from inflammation to immunoregulation. Clin. Med. Insights Blood Disord. 2018;11 doi: 10.1177/1179545X17752325. 1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wataganara T., Leetheeragul J., Pongprasobchai S., Sutantawibul A., Phatihattakorn C., Angsuwathana S. Prediction and prevention of pre-eclampsia in Asian subpopulation. J. Obstet. Gynaecol. Res. 2018;44:813–830. doi: 10.1111/jog.13599. [DOI] [PubMed] [Google Scholar]
- 4.Kwong W., Tomlinson G., Feig D.S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am. J. Obstet. Gynecol. 2018;218:573–580. doi: 10.1016/j.ajog.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Ukah U.V., De Silva D.A., Payne B., Magee L.A., Hutcheon J.A., Brown H., Ansermino J.M., Lee T., von Dadelszen P. Prediction of adverse maternal outcomes from pre-eclampsia and other hypertensive disorders of pregnancy: A systematic review. Pregnancy Hypertens. 2018;11:115–123. doi: 10.1016/j.preghy.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus M., Weber P., Zieleskiewicz L. [Maternal deaths due to hypertensive disorders. Results from the French confidential enquiry into maternal deaths, 2010-2012] Gynécol. Obstét. Fertil. Sénol. 2017;45(12S):S38–S42. doi: 10.1016/j.gofs.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Esteve-Valverde E., Ferrer-Oliveras R., Gil-Aliberas N., Baraldès-Farré A., Llurba E., Alijotas-Reig J. Pravastatin for Preventing and Treating Preeclampsia: A Systematic Review. Obstet. Gynecol. Surv. 2018;73:40–55. doi: 10.1097/OGX.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 8.Sharma K., Singh R., Kumar M., Gupta U., Rohil V., Bhattacharjee J. First-Trimester Inflammatory Markers for Risk Evaluation of Pregnancy Hypertension. J. Obstet. Gynaecol. India. 2018;68:27–32. doi: 10.1007/s13224-017-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui I.A., Jaleel A., Tamimi W., Al Kadri H.M. Role of oxidative stress in the pathogenesis of preeclampsia. Arch. Gynecol. Obstet. 2010;282:469–474. doi: 10.1007/s00404-010-1538-6. [DOI] [PubMed] [Google Scholar]
- 10.Kita N., Mitsushita J. A possible placental factor for preeclampsia: sFlt-1. Curr. Med. Chem. 2008;15:711–715. doi: 10.2174/092986708783885309. [DOI] [PubMed] [Google Scholar]
- 11.Gray K.J., Saxena R., Karumanchi S.A. Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. Am. J. Obstet. Gynecol. 2018;218:211–218. doi: 10.1016/j.ajog.2017.11.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H., He W., Eriksson M., Li J., Holowko N., Chiesa F., Hall P., Czene K. Inherited factors contribute to an inverse association between preeclampsia and breast cancer. Breast Cancer Res. 2018;20:6. doi: 10.1186/s13058-017-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith R.A., Baker P.N. Risk factors, prevention and treatment of hypertension in pregnancy. Minerva Ginecol. 2005;57:379–388. [PubMed] [Google Scholar]
- 14.Lockwood C.J., Krikun G., Caze R., Rahman M., Buchwalder L.F., Schatz F. Decidual cell-expressed tissue factor in human pregnancy and its involvement in hemostasis and preeclampsia-related angiogenesis. Ann. N Y Acad. Sci. 2008;1127:67–72. doi: 10.1196/annals.1434.013. [DOI] [PubMed] [Google Scholar]
- 15.Pijnenborg R., Vercruysse L., Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Brosens I.A., Robertson W.B., Dixon H.G. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J. Pathol. 1970;101 pvi. [PubMed] [Google Scholar]
- 17.Fan M., Xu Y., Hong F., Gao X., Xin G., Hong H., Dong L., Zhao X. Rac1/β-Catenin Signalling Pathway Contributes to Trophoblast Cell Invasion by Targeting Snail and MMP9. Cell. Physiol. Biochem. 2016;38:1319–1332. doi: 10.1159/000443076. [DOI] [PubMed] [Google Scholar]
- 18.Klinge C.M. Non-coding RNAs: long non-coding RNAs and microRNAs in endocrine-related cancers. Endocr. Relat. Cancer. 2018;25:R259–R282. doi: 10.1530/ERC-17-0548. [DOI] [PubMed] [Google Scholar]
- 19.Sajadpoor Z., Amini-Farsani Z., Teimori H., Shamsara M., Sangtarash M.H., Ghasemi-Dehkordi P., Yadollahi F. Valproic acid promotes apoptosis and cisplatin sensitivity through downregulation of H19 noncoding RNA in ovarian A2780 cells. Appl. Biochem. Biotechnol. 2018 doi: 10.1007/s12010-017-2684-0. Published online February 22, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Xiong G., Yang L., Chen Y., Fan Z. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am. J. Transl. Res. 2015;7:2262–2269. [PMC free article] [PubMed] [Google Scholar]
- 21.Shan D., Shang Y., Hu T. Long noncoding RNA BLACAT1 promotes cell proliferation and invasion in human cervical cancer. Oncol. Lett. 2018;15:3490–3495. doi: 10.3892/ol.2018.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q., Jia L., Li X., Guo R., Huang Y., Zheng Y., Li W. Long Noncoding RNAs: New Players in the Osteogenic Differentiation of Bone Marrow- and Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rev. 2018;14:297–308. doi: 10.1007/s12015-018-9801-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Lin S., Li J.L., Ni W., Guo R., Lu J., Kaye F.J., Wu L. CRTC1-MAML2 fusion-induced lncRNA LINC00473 expression maintains the growth and survival of human mucoepidermoid carcinoma cells. Oncogene. 2018;37:1885–1895. doi: 10.1038/s41388-017-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi C., Yang Y., Yu J., Meng F., Zhang T., Gao Y. The long noncoding RNA LINC00473, a target of microRNA 34a, promotes tumorigenesis by inhibiting ILF-2 degradation in cervical cancer. Am. J. Cancer Res. 2017;7:2157–2168. [PMC free article] [PubMed] [Google Scholar]
- 25.Liang X.H., Deng W.B., Liu Y.F., Liang Y.X., Fan Z.M., Gu X.W., Liu J.L., Sha A.G., Diao H.L., Yang Z.M. Non-coding RNA LINC00473 mediates decidualization of human endometrial stromal cells in response to cAMP signaling. Sci. Rep. 2016;6:22744. doi: 10.1038/srep22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X., Li Y., Zhang X., Zuo J., Yang S. The Arabidopsis LSD1 gene plays an important role in the regulation of low temperature-dependent cell death. New Phytol. 2010;187:301–312. doi: 10.1111/j.1469-8137.2010.03275.x. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Chen L., Mu J., Zuo J. LESION SIMULATING DISEASE1 interacts with catalases to regulate hypersensitive cell death in Arabidopsis. Plant Physiol. 2013;163:1059–1070. doi: 10.1104/pp.113.225805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X., Ma C., Zhu Q., Yuan D., Sun M., Gu X., Wu G., Lv T., Song Y. Upregulation of long intergenic noncoding RNA 00673 promotes tumor proliferation via LSD1 interaction and repression of NCALD in non-small-cell lung cancer. Oncotarget. 2016;7:25558–25575. doi: 10.18632/oncotarget.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y., Zhao Y., Wang L., Bohrer L.R., Pan Y., Wang L., Huang H. LSD1 promotes S-phase entry and tumorigenesis via chromatin co-occupation with E2F1 and selective H3K9 demethylation. Oncogene. 2018;37:534–543. doi: 10.1038/onc.2017.353. [DOI] [PubMed] [Google Scholar]
- 30.Adamo A., Sesé B., Boue S., Castaño J., Paramonov I., Barrero M.J., Izpisua Belmonte J.C. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat. Cell Biol. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 31.Hirano K., Namihira M. LSD1 Mediates Neuronal Differentiation of Human Fetal Neural Stem Cells by Controlling the Expression of a Novel Target Gene, HEYL. Stem Cells. 2016;34:1872–1882. doi: 10.1002/stem.2362. [DOI] [PubMed] [Google Scholar]
- 32.Li F., Huarte M., Zaratiegui M., Vaughn M.W., Shi Y., Martienssen R., Cande W.Z. Lid2 is required for coordinating H3K4 and H3K9 methylation of heterochromatin and euchromatin. Cell. 2008;135:272–283. doi: 10.1016/j.cell.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q., Hao Q., Prasanth K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2017 doi: 10.1016/j.tig.2017.11.005. Published online December 14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibb E.A., Brown C.J., Lam W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y., Ge Z., Zhang E., Zuo Q., Huang S., Yang N., Wu D., Zhang Y., Chen Y., Xu H. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8:e3104. doi: 10.1038/cddis.2017.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y., Lian Y., Zhang Y., Huang S., Zuo Q., Yang N., Chen Y., Wu D., Sun L. The long non-coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J. Cell. Mol. Med. 2018;22:1272–1282. doi: 10.1111/jcmm.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Zou Y., Wang W., Zuo Q., Jiang Z., Sun M., De W., Sun L. Down-regulated long non-coding RNA MEG3 and its effect on promoting apoptosis and suppressing migration of trophoblast cells. J. Cell. Biochem. 2015;116:542–550. doi: 10.1002/jcb.25004. [DOI] [PubMed] [Google Scholar]
- 39.Zou Y., Li Q., Xu Y., Yu X., Zuo Q., Huang S., Chu Y., Jiang Z., Sun L. Promotion of trophoblast invasion by lncRNA MVIH through inducing Jun-B. J. Cell. Mol. Med. 2018;22:1214–1223. doi: 10.1111/jcmm.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brünnert D., Piccenini S., Ehrhardt J., Zygmunt M., Goyal P. Sphingosine 1-phosphate regulates IL-8 expression and secretion via S1PR1 and S1PR2 receptors-mediated signaling in extravillous trophoblast derived HTR-8/SVneo cells. Placenta. 2015;36:1115–1121. doi: 10.1016/j.placenta.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Zhang Y., Wang H., Wang J., Zhang Y., Wang Y., Pan Z., Luo S. Aberrantly up-regulated miR-20a in pre-eclampsic placenta compromised the proliferative and invasive behaviors of trophoblast cells by targeting forkhead box protein A1. Int. J. Biol. Sci. 2014;10:973–982. doi: 10.7150/ijbs.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim R., Barker G., Lappas M. TREM-1 expression is increased in human placentas from severe early-onset preeclamptic pregnancies where it may be involved in syncytialization. Reprod. Sci. 2014;21:562–572. doi: 10.1177/1933719113503406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X.H., Zhang H.Y., Lu S., Jiang L.L., Wu J., Yang Y.L., Zhang S.A. MMP-14 aggravates onset of severe preeclampsia by mediating soluble endoglin release. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1209–1215. doi: 10.26355/eurrev_201803_14460. [DOI] [PubMed] [Google Scholar]
- 44.Wallace A.E., Host A.J., Whitley G.S., Cartwright J.E. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am. J. Pathol. 2013;183:1853–1861. doi: 10.1016/j.ajpath.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cross C.E., Tolba M.F., Rondelli C.M., Xu M., Abdel-Rahman S.Z. Oxidative Stress Alters miRNA and Gene Expression Profiles in Villous First Trimester Trophoblasts. BioMed Res. Int. 2015;2015:257090. doi: 10.1155/2015/257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z., Li J.L., Lin S., Cao C., Gimbrone N.T., Yang R., Fu D.A., Carper M.B., Haura E.B., Schabath M.B. cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. J. Clin. Invest. 2016;126:2267–2279. doi: 10.1172/JCI85250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu S., Fu W., Zhang L., Fu K., Hu J., Jia W., Liu G. LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKα. Cell Prolif. 2018;51 doi: 10.1111/cpr.12416. Published online November 20, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q., Zhang Y., Wang S.Z., Wang N., Jiang W.G., Ji Y.H., Zhang S.L. Reduced expression of tissue factor pathway inhibitor-2 contributes to apoptosis and angiogenesis in cervical cancer. J. Exp. Clin. Cancer Res. 2012;31:1. doi: 10.1186/1756-9966-31-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chand H.S., Du X., Ma D., Inzunza H.D., Kamei S., Foster D., Brodie S., Kisiel W. The effect of human tissue factor pathway inhibitor-2 on the growth and metastasis of fibrosarcoma tumors in athymic mice. Blood. 2004;103:1069–1077. doi: 10.1182/blood-2003-06-1930. [DOI] [PubMed] [Google Scholar]
- 50.Hu H., Chen X., Wang C., Jiang Y., Li J., Ying X., Yang Y., Li B., Zhou C., Zhong J. The role of TFPI2 hypermethylation in the detection of gastric and colorectal cancer. Oncotarget. 2017;8:84054–84065. doi: 10.18632/oncotarget.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.