Abstract
Background
The role of miR-181a in the development of cardiac disease and in particular, myocardial fibrosis following myocardial infarction (MI) remains unknown. The aim of this study was to explore the role of miR-181a in myocardial fibrosis in a rat model of MI and the expression of TGF-β receptor III (TβRIII).
Material/Methods
Forty adult male Wistar rats were randomly divided into an MI model group (n=30) and a control group with (n=10). The rat MI model involved ligating the left anterior descending (LAD) coronary artery in the model group; the control group was treated with a sham operation. Cardiac function was assessed using cardiac ultrasound. Myocardial fibroblasts were extracted from the rat hearts and transfected with a miR-mimic or miR-inhibitor, and cell growth was measured using an MTT assay. The level of miR-181a expression was detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blots.
Results
miR-181a expression was significantly increased during the progression of MI (P<0.05). Over-expression of miR-181a was associated with increased deposition of extracellular matrix (ECM) components, collagen I and fibronectin. This effect was reversed with the use of a miR-181a inhibitor (P<0.05). Upregulation of miR-181a suppressed the expression of TGF-β receptor III (TβRIII) by binding with 3′-UTR.
Conclusions
In this rat model of MI, the findings were that miR-181a had a role in the progression of myocardial fibrosis. The findings require further studies to determine whether miR-181a might provide a novel therapeutic target to limit myocardial fibrosis following MI.
MeSH Keywords: Endomyocardial Fibrosis, MicroRNAs, Myocardial Infarction
Background
Myocardial infarction (MI) from coronary artery disease results in more than seven million deaths worldwide each year [1]. Major risk factors for MI are hyperlipidemia, diabetes, hypertension, smoking, and age [2]. During MI, the necrotic cardiac myocytes are replaced with scar tissues formed by fibroblasts, and the resulting impaired cardiac function ultimately leads to progressive heart failure [3]. Previous studies have focused on the molecular mechanism of myocardial cells apoptosis and necrosis. However, there are few reports examining the progression of myocardial fibrosis.
MicroRNAs (miRNAs) are a class of non-coding RNAs approximately, 22 nucleotides in length, which play a role in regulating gene expression by binding to 3′-untranslated region (3′-UTR) of target mRNAs [4,5]. There is increasing evidence from published studies that support the importance of miRNAs as regulators in the development of cardiovascular disease. MicroRNA-181a (miR-181a) is known to play an important role in the development of many cancers [6–9]. Previously published studies have shown that miR-181a impedes the terminal differentiation of myoblasts by negatively regulating the homeobox protein Hox-A11 [10]. Yi-Gang Li et al. found that knockdown of microRNA-181a could decrease the arrhythmogenic effect of skeletal myoblast transplantation in a rat model of MI [11].
Transforming growth factor (TGF)-β receptor III (TβRIII) is a proteoglycan composed of 851 amino acids and is involved in cellular metabolism [12]. As a TGF-β superfamily co-receptor, the role of TβRIII in regulating TGF-β signaling is context-dependent and complex [13,14]. Fei Sun et al. have demonstrated that TβRIII was involved in the reduction of myocardial fibrosis following MI, and in the reduction of collagen deposition, and fibroblast activity, that was induced by simvastatin [15].
The aim of this study was to explore the role of miR-181a in myocardial fibrosis in a rat model of MI, including the role of the expression of TβRIII.
Material and Methods
Experimental animals, and the rat model of myocardial infarction (MI)
Forty male Wistar rats were purchased from the Laboratory Animal Center of Soochow University. The rats had an average age of 2–3 months and weighed between 180–220 gm. The rats in the study were randomly divided into a myocardial infarction (MI) model group (n=30) and a control group (n=10).
The MI model was established by ligating the left anterior descending (LAD) coronary artery in the MI model group; the control group was treated with a sham operation, as previously described [16]. Because the surgical procedure resulted in some loss of rats, the remaining rats in the MI model were randomly divided into a one-week post-MI group A (n=8), a two-week post-MI group B (n=10), and a four-week post-MI group C (n=10). All animal experiments were performed according to institutional, local and national guidelines on animal research and ethics.
Examination of cardiac function
After the induction of MI, the cardiac function indices of each group was measured using ultrasound examination, as follows: left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular end systolic diameter (LVESD), and left ventricular end diastolic diameter (LVEDD).
Cell extraction, culture, and transfection
At the end of the study, the rats were euthanized, and the hearts were removed, placed in culture dishes under aseptic conditions, and rinsed in Hanks’ balanced salt solution (HBSS). The heart was cut into small pieces and washed using water containing five times the volume of 0.05% trypsin at 37°C in 15 ml centrifuge tube for 5 minutes. After mixing, the supernatant was discarded. The precipitated component was repeatedly mixed for approximately 10 minutes with approximately five times the volume of 0.08% trypsin and 0.1% collagenase II. After centrifugation, the precipitation was resuspended in 10 ml Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum which was changed 60–90 minutes later and then inoculated into a culture dish. For transfection, myocardial fibroblasts were seeded into plates and transfected with miRNAs or siRNAs (Gene Pharm, Shanghai, China) mixed with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, MA, USA), according to the manufacturer’s protocols.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using a reverse transcription kit (Takara Biotechnology, Dalian, China). The relative quantity of cDNA was analyzed by quantitative PCR with SYBR green and the ΔΔCT method. β-actin was used as the internal control gene to normalize the target genes. All primers were designed and synthesized by GenePharma (Shanghai, China) and shown as follows:
mir-181a mimic, ACCAUCGACCGUUGAUUGUACC;
miR-181a inhibitor, ACCAUCGACCGUUGAUUGUACC;
negative controls (NC), UUCUCCGAACGUGUCACGUTT;
NC inhibitor, CAGUACUUUUGUGUAGUACAA;
Collagen I, CGAGTATGGAAGCGAAGGT, and
CCACAAGCGTGCTGTAGGT;
Fibronection, CACGGAGGCCACCATTACT, and
CTTCAGGGCAATGACGAGAT;
TβRIII, CGTCAGGAGGCACACACTTA, and
CACATTTGACAGACAGGGCAAT;
β-actin, CACGATGGAGGGGCCGGACTCATC, and
TAAAGACCTCTATGCCAACACAGT.
Relative levels of gene expression were expressed relative to β-actin and calculated using the 2−ΔΔCt method.
Cell proliferation assay
An MTT assay was used to assess cell proliferation status. Cells (3×103) were cultured in 96-well plates and incubated for 24 hours and stained with 0.5 mg/ml MTT for 4 hours. The supernatant was discarded, and 200 μl of dimethylsulfoxide (DMSO) was added to dissolve the precipitates. Samples were measured at 490 nm using an enzyme-linked immunosorbent assay (ELISA) reader.
Luciferase activity assay
The 3′untranslated region (3′-UTR) of TGF-β receptor III (TβRIII) containing miR-181a binding sites was amplified and cloned into the psiCHECK-2 luciferase vector (Promega, USA). Similarly, the mutant 3′-UTR of TβRIII was cloned into the same vector. Myocardial fibroblasts maintained in 96-well plates were co-transfected with a pGL3-control luciferase reporter, pRL-TK vector, and miR-181a mimic, or miR-Control vector, Transfected cells were detected using the Dual-Luciferase Reporter Assay System (Promega) 48 hours later.
Western blot analysis
The cultured cells were washed with ice-cold PBS and lysed in RIPA buffer supplemented with protease inhibitor mixture. After electrophoresis, the protein samples were incubated with the primary antibodies as follows: anti-TβRIII, anti-Collagen I, and anti-fibronectin (1: 1000 dilution). Samples were incubated with secondary antibodies conjugated with horseradish peroxidase (HRP). Bands were quantified using ImageJ software.
Statistical analysis
Results were expressed as the mean ± standard error. The Student’s t-test and ANOVA were performed between different groups. All calculations were performed using SPSS version 17.0 software (IBM Software, Chicago, IL, USA) and GraphPad (vision 6.0, USA). A value of P<0.05 indicated a statistically significant difference.
Results
Expression of miR-181a and fibrotic index in the rat model of myocardial infarction (MI) of each group
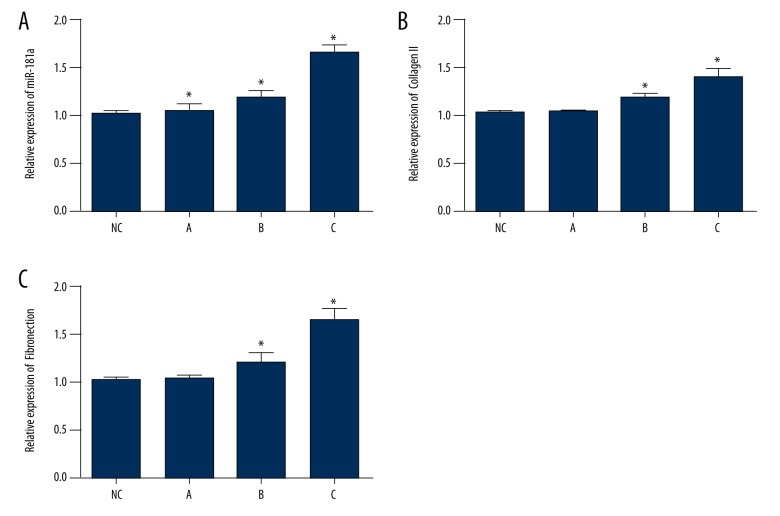
To investigate the effect of miR-181a on the development of myocardial infarction (MI) in a rat model, the expression of miR-181a in cardiac tissues was analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Figure 1A shows that the level of miR-181a mRNA expression was significantly elevated in MI, and that that miR-181a increased over time during the progression of MI. Also, there was a significant increase in the expression of Collagen I and fibronectin mRNA (Figure 1B, 1C).
Figure 1.
Expression of miR-181a and the fibrotic index in a rat model of myocardial infarction (MI model group and control group). (A) The expression of miR-181a mRNA is significantly upregulated in the MI group compared with the control group. (B, C) The change in fibrotic index showed a significant increase in the expression of collagen I and fibronectin mRNA. Data are shown as the mean ±SD. * P 0.05 (Student’s t-test).
Cardiac index of MI in rats of each group
Cardiac function was examined with echocardiography after induction of MI (Table 1). The left ventricular end systolic diameter (LVESD) and left ventricular end diastolic diameter (LVEDD) levels increased significantly over time (P<0.05) and were significantly increased in the MI group compared with the control group (P<0.05). The left ventricular ejection fraction (LVEF) and the left ventricular fractional shortening (LVFS) were significantly reduced over time (P<0.05) and were significantly reduced in the MI group compared with the control group.
Table 1.
Cardiac indexes of MI.
| Group | LVESD (mm) | LVEDD (mm) | LVEF (%) | LVFS (%) |
|---|---|---|---|---|
| NC | 2.46±0.30 | 4.81±0.11 | 75.38±4.57 | 48.70±6.45 |
| A | 3.26±0.16#* | 5.29±0.17#* | 58.89±2.13#* | 38.50±1.83#* |
| B | 3.69±0.12# | 6.51±0.21# | 47.58±2.28# | 43.29±2.82# |
| C | 4.59±0.21#* | 7.51±0.23# | 37.88±2.05# | 38.89±2.57# |
Compared with NC group
P<0.05; compared with the B group
P<0.05. Values are Mean ±SD.
LVESD – leftventricular end systolic dimension; LVEDD – left ventricular end diastolic dimension; LVEF – left ventricular ejection fraction; LVFS – fractional shortening. A – MI after one week; B – MI after two weeks; C- MI after four weeks.
miR-181a promoted the proliferation of myocardial fibroblasts in vitro
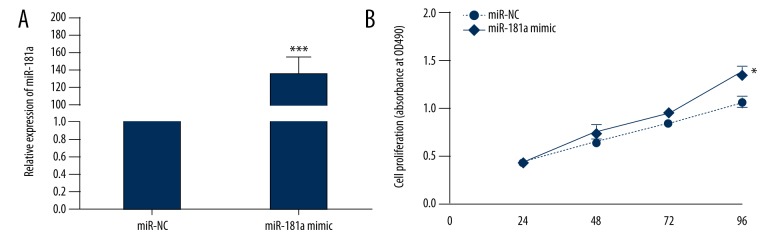
To explore the role of miR-181a in the progression of MI, miR-181a overexpression in myocardial fibroblasts was generated via retrovirus infection. The results of qRT-PCR showed that miR-181a was significantly upregulated in myocardial fibroblasts transduced with a miR-181a mimic when compared with miR-Control (Figure 2A). To explore the role of miR-181a in cell proliferation, an MMT assay was performed to assess the viability of cardiac fibroblasts in MI. The results showed that the growth rate of myocardial fibroblasts transfected with the miR-181a mimic was significantly increased (Figure 2B).
Figure 2.
Effect of miR-181a on cell proliferation detected by the MMT assay. (A) The expression of miR-181a in myocardial fibroblasts treated with miR-Control or the miR-181a (mimic) was detected by quantitative reverse transcription polymerase chain reaction (qRT-PCR). (B) The MMT assay for the proliferation of myocardial fibroblasts transduced, as in A. Data are shown as the mean ±SD. * P<0.05 and *** P<0.001. (Student’s t-test).
miR-181a promoted extracellular matrix formation in vitro
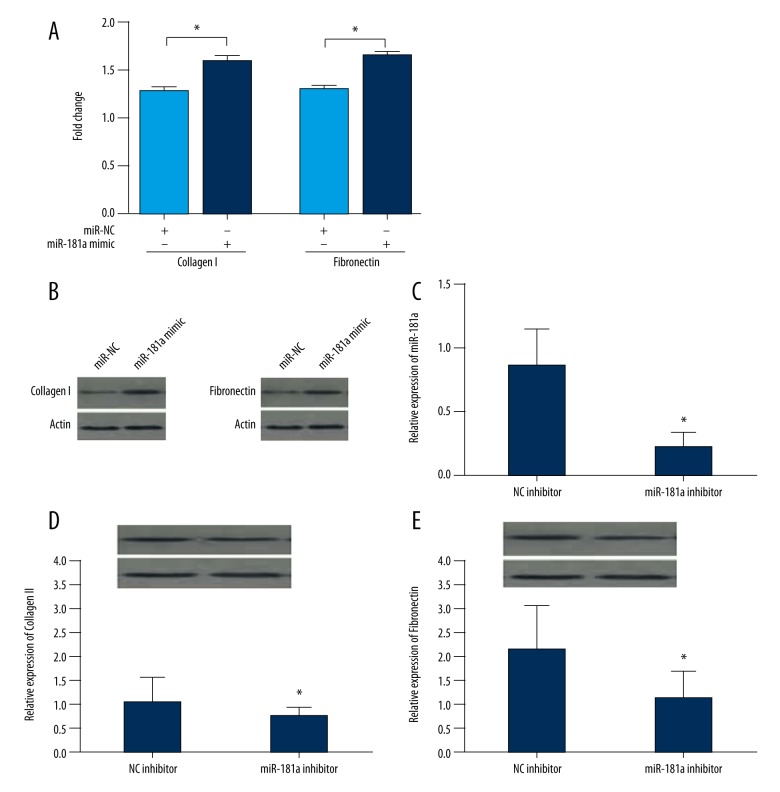
The expression of fibronectin and collagen I was detected using qRT-PCR and Western blot to assess the accumulation of extracellular matrix. The results showed that the over-expression of miR-181a increased the levels of fibronectin and collagen I (Figure 3A). Furthermore, the production of fibronectin and Collagen I was significantly increased in myocardial fibroblasts (Figure 3B). This finding indicated that over-expression of miR-181a increased fibrosis (collagen formation) in myocardial fibroblasts. A small interfering RNA (si-RNA) against miR-181a was successfully designed and assessed using qRT-PCR (Figure 3C). The results of qRT-PCR confirmed the expression of collagen I and fibronectin were significantly reduced in cardiac fibroblasts (Figure 3D, 3E), indicating that miR-181a regulated myocardial fibrosis in vitro.
Figure 3.
Expression of collagen I and fibronectin, the fibrosis index, in myocardial fibroblasts. (A) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) detects the mRNA levels of collagen I and fibronectin in cells treated with miR-Control or the miR-181a (mimic). (B) Western blot measurements of the expression of collagen I and fibronectin in cells transduced, as in A. (C) The expression of miR-181a in cells treated with control-inhibitor and miR-181a inhibitor detected by qRT-PCR. (D, E) Western blots and qRT-PCR for the expression of collagen I and fibronectin in cells treated, as in C. Data are shown as the mean ±SD. * P<0.05 and *** P<0.001. (Student’s t-test).
miR-181a targets with 3′-UTR of TβRIII
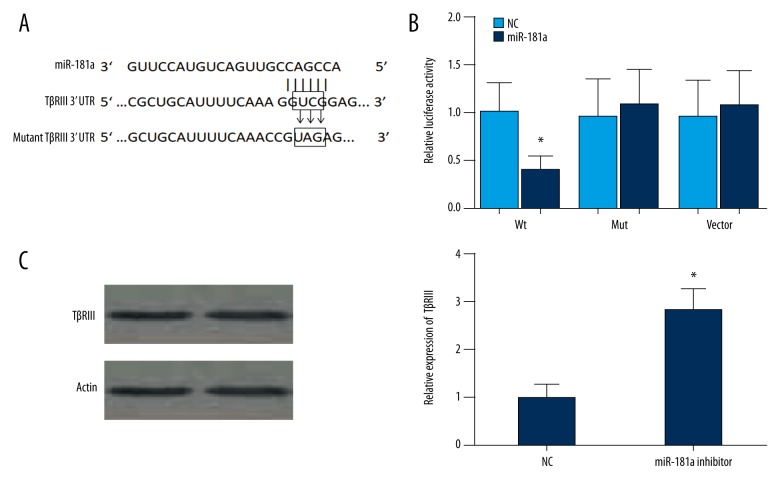
Bioinformatics analysis was used to screen the potential targeting miRNAs binding with miR-181a. The results showed that the 3′-UTR of TβRIII was highly conserved and bound with miR-181a. The 3′-UTR binding sites are shown in Figure 4A. The luciferase reporter assay showed transfection of miR-181a could significantly restrict the relative luciferase activity in myocardial fibroblasts (Figure 4B) (P<0.5), indicating a direct interaction between miR-181a and TβRIII. Furthermore, after cells were transfected with miR-181a inhibitor, TβRIII expression levels were significantly upregulated (Figure 4C). Overall, these results showed that miR-181a suppressed the expression of TβRIII by binding with the 3′-UTR.
Figure 4.
Upregulation of miR-181a suppresses the expression of TGF-β receptor III (TβRIII) by binding with 3′-UTR. Using bioinformatics analysis, 3′ UTR of TGF-β receptor III (TβRIII) is shown to be highly conserved and to bind to miR-181a. The 3′-UTR binding sites are shown as follows: (A) Luciferase reporter assay shows that transfection of miR-181a significantly restricts the relative luciferase activity in myocardial fibroblasts. (B) There appear to be few differences in luciferase activities between the control group and normal cells containing miR-21 in the control group and PTEN mutation 3′-UTR group (* P<0.5). (C) After cells were transfected with miR-181a inhibitor, TGF-β receptor III (TβRIII) expression levels are significantly upregulated. These findings show that miR-181a suppresses the expression of TβRIII by binding with the 3′-UTR.
Discussion
MicroRNAs (miRNAs) are single-stranded, non-coding RNAs, first identified in the nematode, Caenorhabditis elegans in 1993 [17,18]. There is growing evidence that miRNAs play an important role in myocardial cell death and regeneration [19]. miR-181a participates in several gene regulatory processes such as development, differentiation, hematopoiesis, and immune modulation [20,21]. Previous studies have shown miR-181a has varied effects in different types of tumors [22,23]. In cardiac disease, Jianbing Zhu et al. reported that circulating miR-181a levels in patients with myocardial infarction (MI) were significantly changed in a time-dependent manner, indicating the potential value of plasma miR-181a as a novel biomarker in MI [24]. However, the molecular mechanism of miR-181a in MI remains poorly understood.
This current study focused on the role of miR-181a in myocardial fibrosis following MI in a rat model that used coronary artery ligation. The results of this study showed a significant increase in the expression of miR-181a in rats following MI that was time-dependent. Also, the fibrosis indices measured in this study (fibronectin, Collagen I) increased following MI. An MMT assay showed that over-expression of miR-181a accelerated the proliferation of myocardial fibroblasts, and that upregulation of miR-181a promoted the expression of mRNA and the production of the related protein of these indices of fibrosis. These findings were reversed by using knockdown of miR-181a. The data support the role of miR-181a in the promotion of cardiac fibrosis following MI., in this rat model
TGF-β receptor III (TβRIII) is a transmembrane proteoglycan, which acts as a regulator in the TGF-β signaling pathway. In cardiac disease, recent studies have shown that TβRIII plays an important role in reducing myocardial fibrosis by reducing collagen production [16,25]. In this study, the use of bioinformatics analysis showed that miR-181a closely targeted 3′-UTR of TβRIII. These results support that over-expression of miR-181a could lower TβRIII mRNA expression and related protein production, and also support the view that miR-181a may induce myocardial fibrosis through the modulation of TβRIII.
Conclusions
This study investigated the role of miR-181a in the pathogenesis of myocardial infarction (MI) and the development of myocardial fibrosis in a rat model. The findings require further study to determine whether miR-181a might provide a novel therapeutic target to limit myocardial fibrosis following MI.
Footnotes
Source of support: This work was supported by the grant from the Zhejiang Provincial Natural Science Foundation of China (grant No. LY16H050006)
Conflict of interest
All authors declare no conflicts of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Crackower MA, Oudit GY, Kozieradzki I, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110(6):737–49. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 3.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15(3):331–41. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Jing Q, Huang S, Guth S, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120(5):623–34. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Brockhausen J, Tay SS, Grzelak CA, et al. miR-181a mediates TGF-beta-induced hepatocyte EMT and is dysregulated in cirrhosis and hepatocellular cancer. Liver Int. 2015;35(1):240–53. doi: 10.1111/liv.12517. [DOI] [PubMed] [Google Scholar]
- 7.Ma Z, Qiu X, Wang D, et al. MiR-181a-5p inhibits cell proliferation and migration by targeting Kras in non-small cell lung cancer A549 cells. Acta Biochim Biophys Sin (Shanghai) 2015;47(8):630–38. doi: 10.1093/abbs/gmv054. [DOI] [PubMed] [Google Scholar]
- 8.Wei Z, Cui L, Mei Z, et al. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett. 2014;588(9):1773–79. doi: 10.1016/j.febslet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Xu D, Wang Q, et al. LPS induced miR-181a promotes pancreatic cancer cell migration via targeting PTEN and MAP2K4. Dig Dis Sci. 2014;59(7):1452–60. doi: 10.1007/s10620-014-3049-y. [DOI] [PubMed] [Google Scholar]
- 10.Naguibneva I, Ameyar-Zazoua M, Polesskaya A, et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol. 2006;8(3):278–84. doi: 10.1038/ncb1373. [DOI] [PubMed] [Google Scholar]
- 11.Li YG, Zhang PP, Jiao KL, Zou YZ. Knockdown of microRNA-181 by lentivirus mediated siRNA expression vector decreases the arrhythmogenic effect of skeletal myoblast transplantation in rat with myocardial infarction. Microvasc Res. 2009;78(3):393–404. doi: 10.1016/j.mvr.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Casillas F, Cheifetz S, Doody J, et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–95. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 14.Thatcher JD. The TGF-beta signal transduction pathway. Sci Signal. 2010;3(119):tr4. doi: 10.1126/scisignal.3119tr4. [DOI] [PubMed] [Google Scholar]
- 15.Sun F, Duan W, Zhang Y, et al. Simvastatin alleviates cardiac fibrosis induced by infarction via up-regulation of TGF-beta receptor III expression. Br J Pharmacol. 2015;172(15):3779–92. doi: 10.1111/bph.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Zhu L, Li C, et al. Sialyltransferase7A, a Klf4-responsive gene, promotes cardiomyocyte apoptosis during myocardial infarction. Basic Res Cardiol. 2015;110(3):28. doi: 10.1007/s00395-015-0484-7. [DOI] [PubMed] [Google Scholar]
- 17.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 19.Sun T, Dong YH, Du W, et al. The role of microRNAs in myocardial infarction: From molecular mechanism to clinical application. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040745. pii: E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji J, Yamashita T, Budhu A, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50(2):472–80. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekirdag KA, Korkmaz G, Ozturk DG, et al. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy. 2013;9(3):374–85. doi: 10.4161/auto.23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W, Shen H, Liu L, et al. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–66. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Shen ZL, Wang L, et al. Hsa-miR-181a-5p expression and effects on cell proliferation in gastric cancer. Asian Pac J Cancer Prev. 2013;14(6):3871–75. doi: 10.7314/apjcp.2013.14.6.3871. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Yao K, Wang Q, et al. Circulating miR-181a as a potential novel biomarker for diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;40(6):1591–602. doi: 10.1159/000453209. [DOI] [PubMed] [Google Scholar]
- 25.Hermida N, Lopez B, Gonzalez A, et al. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res. 2009;81(3):601–9. doi: 10.1093/cvr/cvn315. [DOI] [PubMed] [Google Scholar]