Abstract
Background
Physical inactivity impairs insulin sensitivity, which is exacerbated with aging. We examined the impact of 2 wk of acute inactivity and recovery on glycemic control, and integrated rates of muscle protein synthesis in older men and women.
Methods
Twenty-two overweight, prediabetic older adults (12 men, 10 women, 69 ± 4 y) undertook 7 d of habitual activity (baseline; BL), step reduction (SR; <1,000 steps.d−1 for 14 d), followed by 14 d of recovery (RC). An oral glucose tolerance test was used to assess glycemic control and deuterated water ingestion to measure integrated rates of muscle protein synthesis.
Results
Daily step count was reduced (all p < .05) from BL at SR (7362 ± 3294 to 991 ± 97) and returned to BL levels at RC (7117 ± 3819). Homeostasis model assessment–insulin resistance increased from BL to SR and Matsuda insulin sensitivity index decreased and did not return to BL in RC. Glucose and insulin area under the curve were elevated from BL to SR and did not recover in RC. Integrated muscle protein synthesis was reduced during SR and did not return to BL in RC.
Conclusions
Our findings demonstrate that 2 wk of SR leads to lowered rates of muscle protein synthesis and a worsening of glycemic control that unlike younger adults is not recovered during return to normal activity in overweight, prediabetic elderly humans.
Clinical Trials Registration
ClinicalTrials.gov identifier: NCT03039556.
Keywords: Sarcopenia, diabetes, metabolism, physical activity
Sarcopenia begins around the fifth decade of life and is associated with an increased risk of type 2 diabetes mellitus (T2DM) (1). Physical inactivity is a risk factor for the development of metabolic dysfunction leading to other negative clinical outcomes (2). Older adults are at greater risk of experiencing periods of acute physical inactivity arising from, for example, hospitalization and ensuing bed rest, which is likely to have important adverse consequences. It is thought that periodic muscle disuse in some cases (3), but not all (4), act in confluence with biological aging to confound metabolic processes in older individuals (5).
Complete muscle disuse (i.e. bed rest or limb immobilization) has deleterious effects on glycemic control in both younger (6,7) and older adults (5,8). Within days of the onset of muscle disuse, there are demonstrable reductions in skeletal muscle mass (9), strength (10), and a rapid onset of peripheral insulin resistance (6). Compared to younger persons, elderly individuals show an impaired recovery (RC) from muscle disuse (11), rending them at greater risk for disuse-induced disease and disability (5). Physical inactivity, as a “milder” form of relative muscle disuse, may also be debilitating in older persons. For instance, hospitalized elderly individuals take only ~650–1,000 steps∙d−1 (12) as do older persons convalescing from illness or surgery (13). Studies have shown that such periods of acute inactivity result in the onset of insulin resistance in younger adults (14–17) but that younger persons recover with a return to daily ambulation (14). To our knowledge, only two studies (18,19) have examined how physical inactivity (daily step reduction [SR]) mimicking hospitalization (12), affects muscle metabolic function in older adults. These studies showed that SR (<1,500 steps.d−1) resulted in losses of muscle mass (18,19), reduction in postprandial (18,19), and postabsorbtive (19) rates of muscle protein synthesis (MPS) (18), as well as declines in insulin sensitivity (18). While important, what these studies (18,19) did not determine was whether these adverse changes are restored with a return to habitual activity. This knowledge gap is of clinical importance given that early remobilization is de rigueur for older individuals convalescing from acute illness with the assumption that it is restorative (20).
We examined the effect of 2 wk of SR (<1,000 steps.d−1) on indices of glycemic control and integrated rates of MPS in overweight, prediabetic older men and women. We also determined if any potential changes in these measures would recover following 2 wk of return to regular activity. We hypothesized that acute physical inactivity would result in an impairment in glycemic control, integrated rates of MPS, muscle mass, and muscle strength (as well as protein/gene expression), but that these changes would be recovered within 2 wk of returning to regular activity.
Research Design and Methods
Participants
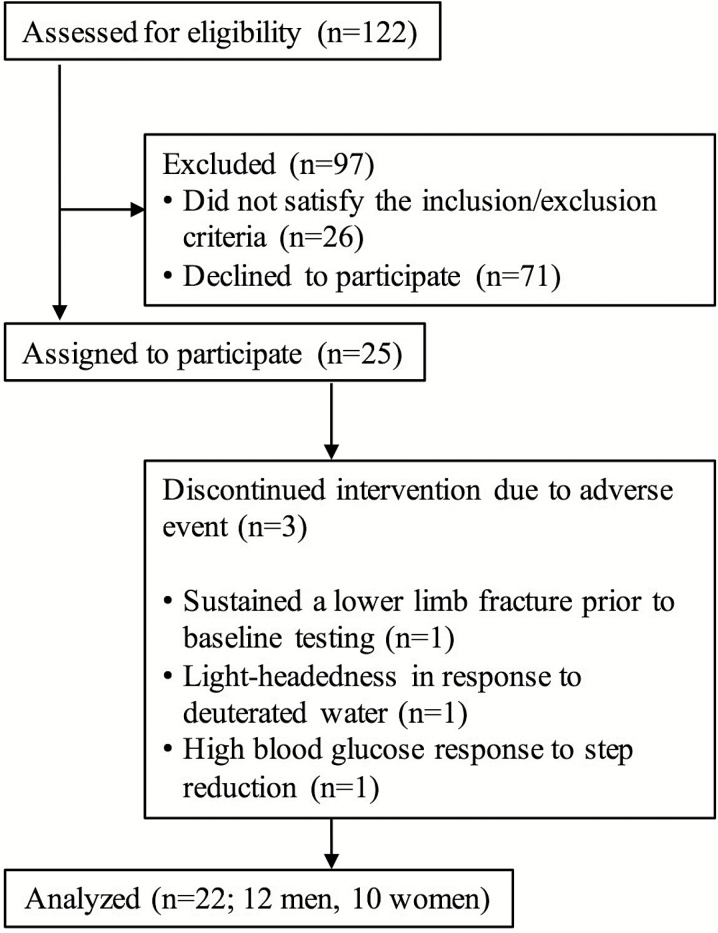
A Consolidated Standards of Reporting Trials participant flowchart can be seen in Figure 1 and participants’ characteristics are presented in Table 1. Twenty-two, older adults (12 men, 10 women, aged 65–79 y) were recruited from the local Hamilton region and surrounding area. This sample size has previously been shown to induce decrements in glycemic control and MPS in response to physical inactivity in our laboratories (18,19). Prior to study inclusion, participants were screened to ensure that they met the following eligibility criteria: nondiabetic (fasting glucose <7.0 mM) (21), nonsmoking, and free from chronic disease. Participants were excluded if they regularly consumed nonsteroidal anti-inflammatory drugs or medication for cholesterol management or if they were considered not to be moderately active (achieving <3,500 steps∙d−1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) participant flowchart.
Table 1.
Participant Characteristics and Daily Step Count
| Baseline | Step Reduction | Recovery | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Age, y | 69 ± 3 | 70 ± 5 | – | – | – | – |
| Mass, kg | 82.6 ± 16.1 | 70.3 ± 13.0 | 83.2 ± 16.7 | 70.3 ± 12.9 | 83.6 ± 16.9 | 70.3 ± 13.0 |
| BMI, kg m−2 | 27.2 ± 4.6 | 27.7 ± 5.1 | 27.6 ± 4.7 | 27.6 ± 5.1 | 27.7 ± 4.7 | 27.6 ± 5.2 |
| Pedometer Steps d−1 | 7,880 ± 3,800a | 6,585 ± 2,370a | 973 ± 83b | 1,018 ± 116b | 7,895 ± 4,323a | 5948 ± 2759a |
| SenseWear Steps d−1 | 7,027 ± 3,738a | 4,563 ± 1,478a | 1,295 ± 1,022b | 1,012 ± 430b | 6,152 ± 4,070a | 4,142 ± 1,899a |
| PA ≥ 3 METS, min d−1 | 101 ± 86a | 27 ± 16a | 16 ± 14b | 5 ± 4b | 95 ± 84a | 25 ± 18a |
| DEE, kJ d−1 | 10,982 ± 2,118a | 7,984 ± 917a | 8,827 ± 1,296b | 7,061 ± 656b | 10,683 ± 2,029a | 7,577 ± 758a |
Note: BMI = body mass index; DEE = daily energy expenditure; METS = metabolic equivalent; PA = physical activity. Data expressed as Mean ± SD, Means that do not share a letter are significantly different (p < .05), n=22.
Study Approval
The study was approved by the Hamilton Integrated Research Ethics Board (REB 14–609) and adhered to the ethical standards outlined by the Canadian tri-council policy statement regarding the use of human participants in research (22). All testing visits occurred within a 5-wk period within April 2014 and May 2016.
Experimental Outline
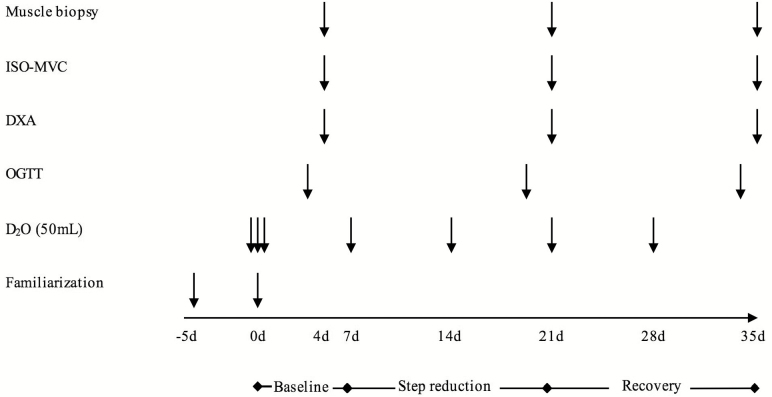
A schematic overview of the experimental design can be seen in Figure 2. The study consisted of three phases: Baseline (BL), SR, and RC. Participants reported to the laboratory following a ~10-h overnight fast on eight separate occasions. On the first two occasions, participants performed familiarization trials of muscular strength. After these initial assessments, participants underwent 7 d of BL monitored normal physical activity with an oral glucose tolerance test (OGTT) on the last day. On the next day, body composition, a skeletal muscle biopsy, and measurement of isometric maximal voluntary contraction. Participants then undertook a 14-d period of SR (<1,000 steps d−1), followed by a return to habitual step-count for the last 14 d of the study as RC. All the measures (OGTT, body composition, muscle biopsy, ISO-MVC) were repeated in sequential order on the last 2 d of the SR and RC phases.
Figure 2.
Schematic diagram of the experimental design. Arrows indicate timing of intervention. DXA = dual-energy X-ray absorptiometry; ISO-MVC = isometric maximal voluntary contraction; OGTT = oral glucose tolerance test.
Daily Step-Count, Dietary Intake, Daily Energy Expenditure
Participants’ daily step-count was monitored using a hip-placed pedometer unit (Piezo SC-StepX Health System, StepsCount, Deep River, Ontario, Canada) that was internally validated using a SenseWear armband accelerometer (BodyMedia, Pittsburgh, Pennsylvania). The SenseWear armband accelerometer (BodyMedia) was also used to estimate daily energy expenditure. Our rationale for the <1,000 steps d−1 threshold in the present study was based on data (12,20) showing that elderly individuals hospitalized for an acute illness only take 650–1,000 steps d−1 regardless of their pre-admittance step count. For the last 3 d of each phase (i.e. during testing), participants were provided with all foods that consisted largely of flash-frozen and prepackaged foods (Heart to Home, Hamilton, Ontario, Canada) with a daily macronutrient distribution of 15% protein (1.0 g kg−1 body mass−1), 30% fat, and 55% carbohydrate of total energy. Daily energy requirements of each participant were calculated using the Harris-Benedict equation (23) with an activity factor based on the results of the International Physical Activity Questionnaire (24).
Body Composition
Body composition was measured using dual-energy x-ray absorptiometry (DXA) body scanning (Lunar iDXA; GE Medical Systems, Madison, Wisconsin), calibrated using a three-compartment Universal Whole Body DXA Phantom (Orthometrix, Naples, Florida). Body scans were used to determine total body fat mass; total body fat- and bone-free mass (FFM); and FFM in the arm, leg, and abdominal regions.
Glucose Regulation
Regulation of blood glucose was assessed using an OGTT. Participants reported to the laboratory following a ~10-h overnight fast. Participants rested in a supine position on a bed, and an intravenous catheter was inserted into an antecubital vein. Immediately following the initial blood draw, participants ingested 75 g glucose in 296 mL (Trutol, Thermo-Scientific, Toronto, Ontario, Canada) before additional blood samples were obtained at 15, 30, 45, 60, 75, 90, and 120 min. Blood samples were subsequently centrifuged at 4,000g for 10 min at 4°C. Plasma was stored at −80°C until further analysis.
Isotope Protocol
To increase deuterium (2H) enrichment in total body water to ~1%, participants consumed 150 mL of 70% D2O (Cambridge Isotope Laboratories, Tewksbury, Massachusetts) on the first day of the BL phase. To maintain the ~1% enrichment in total body water, participants consumed 50-mL doses every 7 d until the end of the study (Figure 2). Total body water 2H2O enrichments were used as a surrogate for labeling of plasma alanine.
Blood Analysis
Plasma glucose and insulin concentrations were measured as described previously (25). Commercially available enzyme-linked immunosorbent assays were used to determine concentrations of interleukin 6, glycated hemoglobin, tumor necrosis factor-alpha (Thermo-Scientific), and C-reactive protein (Cayman Chemical, Ann Arbor, Michigan) by following the manufacturers’ instructions. Intra-assay coefficient of variation was <5% for all blood analysis.
Maximal Isometric Voluntary Contraction
Participants performed unilateral (nonbiopsied, dominant leg) isometric contractions at a 60° angle to measure maximal knee extensor torque using a dynamometer (Biodex System 3; Biodex Medical Systems, Shirley, New York) as previously described in detail (19).
For MPS, immunohistochemistry, microarray analysis, immunoblotting, and calculations. See Supplementary methods.
Statistics
Except where indicated, all statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, New York). Data were checked for normality using the Shapiro-Wilk test and analyzed using two-way between–within mixed analysis of variance with sex and time as between and within factors. When no effect of sex was detected, groups were collapsed and are presented as a single data set analyzed by one-way analysis of variance with the effects across time. Tukey’s post hoc test was used to evaluate significant effects. In all analyses, p < .05 was considered statistically significant.
Results
Step Count, Energy Expenditure, and Dietary Intake
In response to SR, participants’ daily step count was significantly reduced by ~70% from BL at SR (p < .01) and was returned to BL levels in RC (Table 1). Daily energy expenditure exceeding three metabolic equivalents mirrored the changes in daily step count (p < .01, Table 1). Daily dietary intake prior to the 3-d control period was 53 ± 5% carbohydrate, 30 ± 6 fat, 17 + 3% protein [1.0 ± 0.3 g.kg−1], 2,033 ± 402 kCal.
Glycemic Control
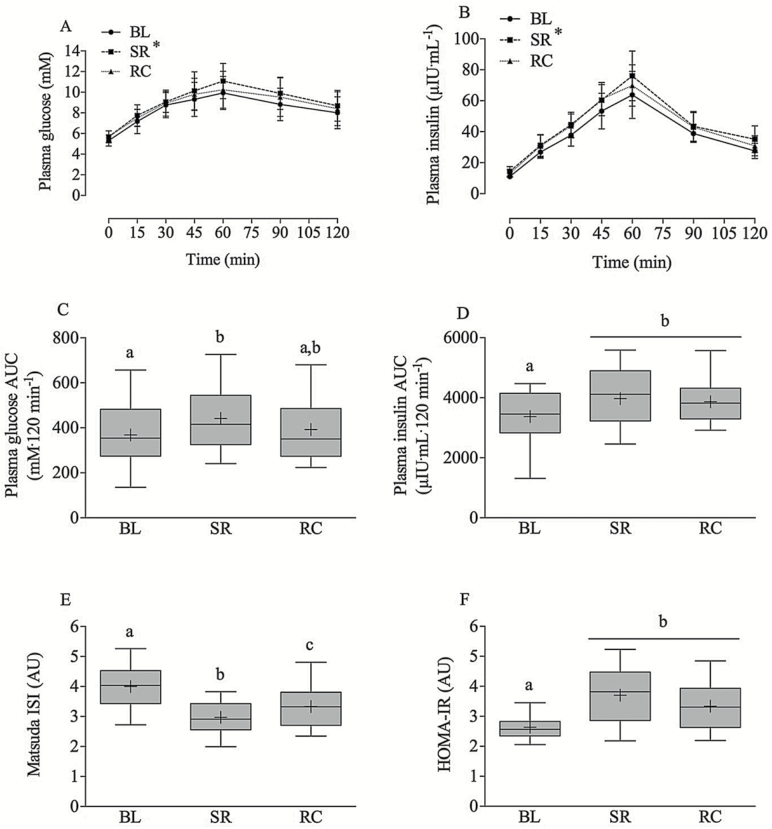
Plasma glucose and insulin concentrations were significantly elevated from BL at SR (p < .01) and were not fully recovered from SR at RC (Figure 3A and B). Plasma glucose area under the curve was significantly elevated from BL at SR (p < .01) and was not fully recovered from SR at RC but was also not significantly different from BL (p < .05, Figure 3C). Insulin area under the curve increased from BL to SR (p < .05) and was still elevated in RC (p < .05, Figure 3D). Matsuda insulin sensitivity index decreased (3.9 ± 0.7 to 2.9 ± 0.4, P < 0.05, Figure 3E) and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) increased (2.6 ± 0.4 to 3.8 ± 0.9, p < .05, Figure 3F) from BL to SR and neither returned to BL levels in RC. Fasted plasma glucose and insulin concentrations as well as glucose concentrations at 120 min post OGTT were also elevated from BL at SR and were not fully returned to BL concentrations in RC (Table 2).
Figure 3.
Plasma glucose (A and C) and insulin (B and D) concentrations over time and AUC, Matsuda insulin sensitivity index (ISI) (E) values in response to an OGTT, and HOMA-IR (F) at baseline (BL), step reduction (SR), and recovery (RC). Boxes represent 25th–75th quartiles, whiskers represent maximum and minimum, horizontal line represents median, cross represents mean. Means that do not share a letter are significantly different (p < .05), and * denotes statistical difference from all other time points. n =22. AUC = area under the curve; OGTT = oral glucose tolerance test.
Table 2.
Plasma Metabolites
| Baseline | Step Reduction | Recovery | |
|---|---|---|---|
| Glucose fasting, mM | 5.3 ± 0.5a | 5.7 ± 0.6b | 5.7 ± 0.5b |
| Glucose 60 min, mM | 9.9 ± 1.6a | 11.1 ± 0.4b | 10.3 ± 0.4a |
| Glucose 120 min, mM | 8.8 ± 1.5a | 9.2 ± 1.5b | 8.7 ± 1.8a,b |
| Glucose peak, mM | 10.4 ± 1.4a | 11.4 ± 1.6b | 10.7 ± 1.6a |
| Insulin fasting, μIU∙mL−1 | 11.1 ± 1.5a | 14.5 ± 3.1b | 13.2 ± 2.3b |
| HBA1c, % | 5.90 ± 0.30a | 5.91 ± 0.32a | 5.94 ± 0.33b |
| HBA1c, mM | 41.1 ± 3.3a | 41.3 ± 3.4a | 41.6 ± 3.6b |
| TNF-α, pg∙mL−1 | 11.8 ± 2.8a | 15.4 ± 4.6b | 14.3 ± 3.8c |
| IL-6, pg∙mL−1 | 8.3 ± 3.1a | 10.8 ± 4.4b | 10 ± 3.4b |
| CRP, µg∙mL−1 | 1.1 ± 0.4a | 1.6 ± 0.7b | 1.4 ± 0.8 a,b |
Note: CRP = C-reactive protein; HBA1c = glycated hemoglobin; IL-6 = interleukin-6; TNF-α = tumor necrosis factor alpha. Data expressed as Mean ± SD. Means that do not share a letter are significantly different (p < .05), n=22.
Muscle Protein Synthesis
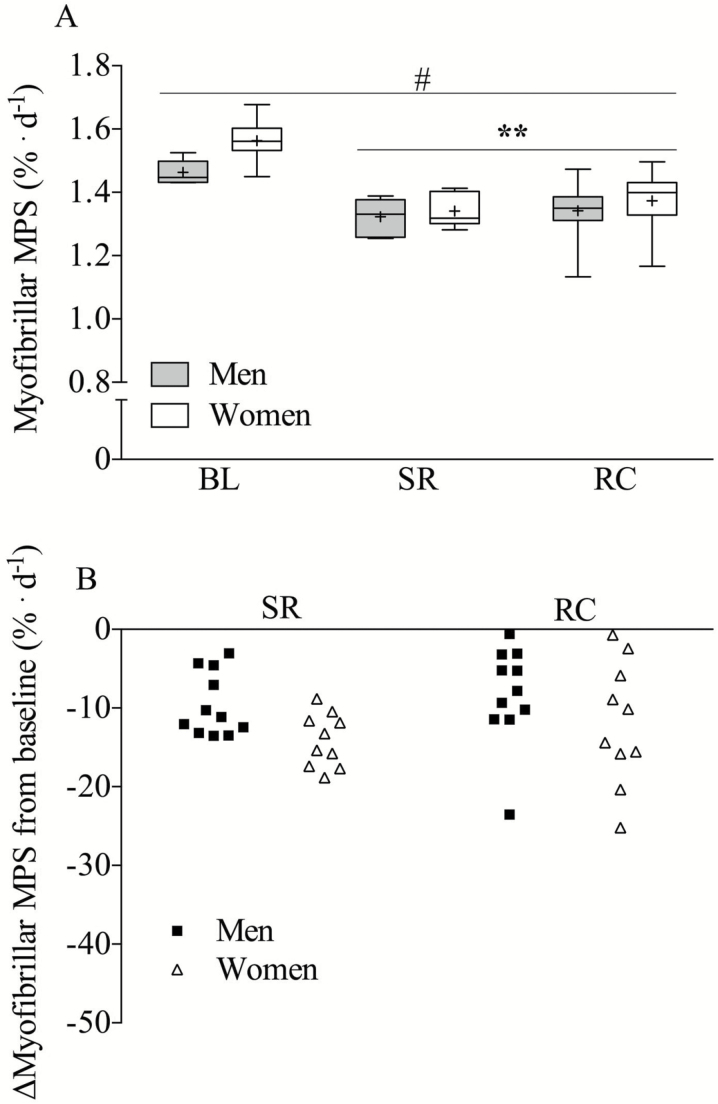
Integrated rates of MPS were reduced from BL at SR (BL 1.51 ± 0.07 to SR 1.33 ± 0.05% d−1, p < .05) and were not restored at RC (1.34 ± 0.14% d−1, Figure 4A). Women had significantly higher rates of integrated MPS across all time points compared to men (p < .05). Isotopic enrichments of salivary 2H2O across the duration of the study are shown in Supplementary Figure 1.
Figure 4.
Integrated rate of myofibrillar protein synthesis (MPS) at baseline (BL), step reduction (SR), and recovery (RC) in men (tin) and women (white) (A). Individual percentage (%) change in MPS from BL in men (black boxes) and women (open triangles). Panel A—Boxes represent 25th–75th quartiles, whiskers represent maximum and minimum, horizontal line represents median, cross represents mean. Panel B—Mean (center line) ±95% confidence interval. #Significantly different from men (main effect, p < .05), n=22 (12 men, 10 women). ** Significantly different from BL for both sexes.
Circulating Inflammatory Markers
Concentrations of circulating inflammatory markers are shown in Table 2. Circulating plasma tumor necrosis factor-alpha, interleukin 6, and C-reactive protein concentrations were significantly increased from BL at SR (p < .01) and still elevated above BL in RC (p < .01).
Body Composition
Body mass index, total body fat percentage, and total lean mass remained unaltered throughout the duration of the protocol (Table 3). Leg lean mass declined −0.6 ± 2%, but this reduction was not significant (p = .13).
Table 3.
Body Composition
| Baseline | Step Reduction | Recovery | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Total BF, % | 29.2 ± 10.4 | 42.9 ± 7.7 | 29.2 ± 10.3 | 42.8 ± 7.8 | 29.1 ± 10.1 | 42.6 ± 7.7 |
| Total FM, kg | 24.6 ± 12.8 | 30.2 ± 9.8 | 24.7 ± 12.8 | 30.2 ± 9.8 | 24.7 ± 12.8 | 30.1 ± 9.7 |
| Total body FFM, kg | 54.9 ± 6.3 | 38.1 ± 5.0 | 55.2 ± 6.2 | 38.1 ± 4.7 | 55.6 ± 6.3 | 38.2 ± 4.9 |
| Trunk FM, kg | 14.6 ± 8.2 | 15.2 ± 5.8 | 14.6 ± 8.2 | 15.1 ± 5.9 | 14.7 ± 8.1 | 15.0 ± 5.7 |
| VAT, cm3 | 1,697 ± 1,225 | 1,093 ± 641 | 1,685 ± 1,185 | 1,105 ± 693 | 1,683 ± 1,148 | 1,120 ± 624 |
| ALM, kg | 25.3 ± 3.5 | 16.8 ± 2.1 | 25.1 ± 3.4 | 16.8 ± 2.1 | 25.5 ± 3.6 | 17.0 ± 2.2 |
| Leg LM, kg | 18.7 ± 2.7 | 13.0 ± 1.5 | 18.6 ± 2.7 | 13.0 ± 1.5 | 18.9 ± 2.9 | 13.2 ± 1.6 |
| SMI, kg/m2 | 8.4 ± 0.8 | 6.6 ± 0.7 | 8.3 ± 0.8 | 6.6 ± 0.7 | 8.5 ± 0.8 | 6.7 ± 0.8 |
Note: ALM = appendicular lean mass; BF = body fat; BMD = bone mineral density; FFM = fat-free mass; FM = fat mass; LM = lean mass; SMI = skeletal muscle mass index; VAT = visceral adipose tissue. Data expressed as Mean ± SD, n=22.
Muscle Fiber Cross-Sectional Area, Distribution, and Strength
There were no significant changes in either type1 fiber cross sectional area, type 2 fiber cross sectional area, or fiber type distribution (type 1 vs. type 2) at any time point (Table 4). Strength (isometric maximal voluntary contraction) was not altered from BL at SR or RC (Table 4).
Table 4.
Muscle Fiber Cross-sectional Area, Distribution, and Leg Strength
| Baseline | Step Reduction | Recovery | |
|---|---|---|---|
| Type 1 fCSA µm2 | 4,243 ± 778 | 4,273 ± 921 | 4,381 ± 642 |
| Type 2 fCSA µm2 | 4,040 ± 1,478 | 3,873 ± 470 | 3,952 ± 1,242 |
| Type 1 distribution (%) | 40 ± 15 | 40 ± 13 | 40 ± 14 |
| Type 2 distribution (%) | 57 ± 15 | 58 ± 11 | 58 ± 13 |
| ISO-MVC (N∙m) | 139 ± 45 | 143 ± 48 | 141 ± 47 |
Note: fCSA = fiber cross-sectional area; ISO-MVC = isometric maximal voluntary contraction. Data expressed as Mean ± SD, n=22.
Gene and Mitochondrial Complex Protein Expression
A total of 36,245 Illumina HT12v4 microarray probes passed quality control filters, and 47 probes were significantly different in mRNA abundance between BL and SR (p < .05; Figure 5 and Supplementary Table 1). Three probes hybridized to loci withdrawn from the official genome annotation (excluded from Figure 5) and two additional pairs of probes hybridized to the same loci, resulting in differential expression detected for 41 protein-coding genes and 1 antisense RNA encoding gene. DAVID functional annotation indicated that a large fraction of differentially expressed genes were involved in mitochondrial function but additionally detected enrichment of terms for oxoglutarate metabolism, oxidative phosphorylation, and other diseases (Supplementary Table 2). There was no change in the content of mitochondrial protein complexes (Supplementary Figure 2).
Figure 5.
Heat map of gene expression patterns for significant microarray probes (excluding three mapping to loci withdrawn from the official genome annotation), green reflecting downregulation, red reflecting upregulation, and black reflecting no change. The left column reflects changes between baseline (BL) and step reduction (SR), with fold-change indicated. The second column reflects changes between SR and recovery (RC), with both fold-change percent recovery to BL indicated (RC/BL). Gene products involved with the mitochondria, as indicted by GO Slimmer analysis, are at the top of the heat map. Genes are additionally labeled by DAVID prediction of enriched Gene Ontology and KEGG terms: (A) mitochondrial ATP synthesis, (B) 2-oxoglutarate metabolic process, (C) oxidative phosphorylation, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, (D) mitochondrion, (E) nucleolus, and (*) KEGG metabolic pathways.
Discussion
We report that 2 wk of SR (daily steps <1,000 d−1) impaired glycemic control and resulted in declines in integrated rates of MPS in overweight, prediabetic older adults. Importantly, these outcomes were not recovered upon returning, for 2 wk, to habitual levels of physical activity. The detrimental impact of an abrupt period of physical inactivity on glycemic control and integrated rates of MPS was associated with elevated concentrations of inflammatory markers. Our study represents a relevant clinical observation showing that prediabetic older persons are susceptible to inactivity-induced worsening of an insulin-resistant phenotype, as is observed in healthy older and younger persons (14–17). Uniquely, we show that the reversal of this phenotype, which occurs in younger adults (14), is impaired on return to normal activity in our population. Given that age per se is an independent risk for the development of T2DM (26), our findings are highly relevant and suggest that in prediabetic older adults to recover metabolic health and prevent further declines due to periods of inactivity proactive strategies such as prescribed activity/exercise and/or pharmaceutical intervention may be warranted.
Previous reports have shown that periods (≤2 wk) of SR induced a decline in insulin sensitivity and an increase in insulin resistance in both younger (14,16,17,27) and older adults (18). Our study provides an important extension to these findings showing a 35% decrease in Matsuda insulin sensitivity index and a 23% increase in HOMA-IR in response to SR that were not restored to preintervention levels with a return to habitual activity in overweight, prediabetic older adults. Our participants’ fasting plasma glucose concentrations were significantly increased from BL at SR and remained elevated at RC. Moreover, while glucose area under the curve during the OGTT returned to BL levels during RC, this reduction was accompanied by an elevation in insulin secretion. We posit that the increase in insulin secretion was a compensatory adaptation to mitigate hyperglycemia, which has been proposed to be a key step in the pathogenesis of T2DM (28). It is likely that insulin resistance at the level of skeletal muscle contributed in part to the insulin-resistant state as seen by the reduction in the Matsuda insulin sensitivity index and in accordance with studies employing euglycemic clamps in response to SR (16,17). However, unlike work in younger adults (16,17), we identified an increase and failed RC in HOMA-IR consistent with impaired hepatic insulin sensitivity. We do acknowledge that the HOMA-IR may not be able to fully discriminate between central, hepatic, or peripheral insulin resistance. Future work utilizing a similar design to that of our study with glucose/insulin clamps may therefore add to our findings. Nevertheless, our findings indicate that in older adults a 2-wk period of physical inactivity impairs glycemic control in both skeletal muscle and liver, potentially rendering older individuals at greater risk of developing T2DM and associated conditions.
Prediabetes increases the risk for T2DM and cardiovascular disease. The American Diabetes Association criteria for diagnosis of prediabetes are fasted glucose concentrations of 5.6–6.9 mM, glycated hemoglobin of 5.7%–6.4%, and/or 2-h post OGTT glucose concentrations of 7.8–11 mM. Considering these guidelines, at BL, all our participants are classified as prediabetic. The prediabetic state of our participants may have increased the susceptibility toward the development of a full T2DM phenotype in response to reduced activity that may not have been observed in individuals who exhibited otherwise normal glycemic control. However, it is important to acknowledge that SR resulted in only one participant breaching the classification for full T2DM (2-h OGTT glucose concentration of 16 mM) suggesting that our data are representative of a preclincally relevant shift in dysglycemia as opposed to the development of overt T2DM. Nevertheless, as we detected a significant worsening of glycemic control in response to SR and that up to 50% of adults over 65 y in the United States and at least 22% of adults in Canada are classified as prediabetic, we argue that our data are highly relevant for a significant portion of the North American population.
Skeletal muscle is the largest site of postprandial glucose disposal the size and quality of which is dictated by fed and fasted-state rates of MPS. A significant finding of the present study was that 2 wk of SR significantly lowered integrated rates of MPS and that these rates were not fully recovered to BL levels after return to habitual step count. Interestingly, despite a higher mean integrated rate of MPS at BL (1.56 ± 0.06% vs 1.47 ± 0.04% d−1) and relative mean decline from BL to SR (~14 vs 9%; Figure 4B) in women, there was no significant sex-based interaction in changes in MPS over time. The sex-specific differences in integrated rates of MPS align with a report using stable isotope amino acid infusions (29). This factor may be relevant considering that women had lower absolute lean mass compared to men meaning that multiple acute bouts of inactivity may render them at greater risk for disuse-induced muscle atrophy over time (11). Concomitant with the reduction in rates of MPS in response to SR was accompanied by a small but signficant increase in circulating inflammatory markers in both men and women. The decline in MPS coupled with the emergence of a hyperglycemic inflamed state may well represent a pernicious confluence of factors that precipitates reductions in muscle mass and metabolic health over time (5,30).
Contrary to our initial hypothesis, the reduction in rates of MPS in response to SR did not lead to a detectable reduction in DXA-measured FFM. One interpretation of this finding is that there may also have been an adaptive decline in rates of muscle proteolysis, but we lack any experimental evidence for this supposition. Moreover, a decline in protein breakdown seems unlikely since previous work has shown significant declines in both leg lean and total FFM with SR (<1,500 d−1 for 2 wk) (18,19) and that our microarray data failed to detect any significant changes in proteolytic markers. It is interesting to note that the starting lean mass of our participants was significantly (p < .01) lower than that of the participants in the studies of Devries et al. (19). and Breen et al. (18). Thus, it is possible that the greater absolute amount of lean tissue in previous work (18,19) rendered the participants more susceptible to losing lean tissue in response to SR compared to those of the present investigation. Evidence for this assertion arises from data demonstrating that such a pattern of response has been observed when comparing younger and older participants undergoing muscle disuse (11). It is also possible that the decrease in integrated rates of MPS preceded any detectable changes in fiber cross-sectional area using histochemical approaches or FFM using DXA.
Our unbiased microarray analysis of muscle gene expression identified subtle but statistically significant, false-discovery rate adjusted, alterations in the expression of mitochondrial-related genes that did not fully return to BL levels at RC (Figure 5). Such data are of particular relevance as mitochondrial proteins have been proposed to play a key role in the development of muscle atrophy and insulin resistance (31) in a variety of experimental settings. We also probed for changes in the content of mitochondrial protein complexes using immunoblotting but did not detect changes response to SR. Our findings contrast with a recent report in which significant declines in mitochondrial protein content were observed with 1 wk of bed rest in healthy young volunteers (6), although the model of complete disuse seen in bed rest is complete disuse versus that of the reduced steps to an extent that we propose explains the different findings. A limitation of the present investigation is that we did not include a parallel young comparator group precluding our ability to make direct young versus old comparison. However, complete RC of insulin sensitivity and lower glucose area under the curve has been reported in younger adults 16 d after SR (14). It is also possible that had we extended our RC period (e.g. >2wk), we may have observed broader or even full RC of our measured variables. Nevertheless, that our older subjects did not fully recover is, we believe, indicative of an age-related difference. We also acknowledge that no measurements of glucose-specific transporters (i.e. GLUT-4) were made in the present investigation, a factor that may be important in future research may wish to consider.
In conclusion, we show that 2 wk of acute physical inactivity, akin to that experienced during hospitalization or during convalescence from illness, impairs both postprandial and postabsorptive glycemic and induced the onset of a diabetic phenotype in overweight, prediabetic older adults. We also show that 2 wk of SR induced a decline in integrated rates of MPS. Critically, neither decrements in glycemic control nor rates of MPS were fully recovered following 2 wk of return to normal ambulation. We propose that our data provide a case for the development and implementation of feasible strategies to mitigate declines in, and expedite RC of, metabolic function in older adults following acute periods of inactivity.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This study was supported by grants from the Canadian Diabetes Association [OG-3-14-4489-SP], Canadian Institutes for Health Research, and the McMaster University Faculty of Science Interdisciplinary Research Fund awarded to S.M.P. S.M.P. holds a Canada Research Chair and acknowledges that support. T.S. holds a Canadian Institutes of Health Research graduate scholarship. A.G.M. holds a Cisco Research Chair in Bioinformatics, supported by Cisco Systems Canada, Inc. B.A.L. was supported by a M.G. DeGroote Institute for Infectious Disease Research Summer Student Fellowship. Computer resources were supplied by the McMaster Service Lab and Repository computing cluster.
Author Contributions
C.M., M.V.A., T.S., B.K.S., G.S., and S.M.P. designed the study procedures and analysis. C.M., T.S., and M.V.A., conducted the study. C.M., T.S., A.J.H., R.M., B.A.L., A.G.M., A.R.R., and B.K.S., performed the analysis. A.R.R., designed the computational infrastrcture for microarray analysis. C.M., drafted the manuscript and all authors edited and approved the final version of the manuscript. C.M. and S.M.P., take full responsibility for the integrity of the data and accuracy of the analysis.
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgments
The authors would like to thank all the participants for their time and willingness to participate in this investigation as well as Dr. Ben Bolker, and Brian Alcock for their assistance with the statistical analyses.
References
- 1. Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol. 2012;2012: 320482. doi: 10.1155/2012/320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59: 2527–2545. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70: 57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 4. Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32: 1547–1549. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13: 34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65: 2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 7. Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985). 2011;111: 1201–1210. doi: 10.1152/japplphysiol. 00698.2011. [DOI] [PubMed] [Google Scholar]
- 8. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12: 898–906. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 9. Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf). 2014;210: 600–611. doi: 10.1111/apha.12190. [DOI] [PubMed] [Google Scholar]
- 10. English KL, Mettler JA, Ellison JB, et al. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103: 465–473. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suetta C, Hvid LG, Justesen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985). 2009;107: 1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 12. Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59: 91–95. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell KE, von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging. 2016;5: 33–41. doi: 10.14283/jfa.2016.78. [DOI] [PubMed] [Google Scholar]
- 14. Knudsen SH, Hansen LS, Pedersen M, et al. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J Appl Physiol (1985). 2012;113: 7–15. doi: 10.1152/japplphysiol.00189.2011. [DOI] [PubMed] [Google Scholar]
- 15. Olsen MH, Hansen TW, Christensen MK, et al. Impact of the metabolic syndrome on the predictive values of new risk markers in the general population. J Hum Hypertens. 2008;22: 634–640. doi: 10.1038/jhh.2008.40. [DOI] [PubMed] [Google Scholar]
- 16. Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012;44: 225–231. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krogh-Madsen R, Thyfault JP, Broholm C, et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985). 2010;108: 1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 18. Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98: 2604–2612. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 19. Devries MC, Breen L, Von Allmen M, et al. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep. 2015;3: e12493. doi: 10.14814/phy2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170: 1942–1943. doi: 10.1001/archinternmed.2010.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 (Suppl 1): S81–90. [DOI] [PubMed] [Google Scholar]
- 22. Canadian Institutes of Health Research NSERC, Social Sciences and Humanities Research Council of Canada. Tri-council policy statement: ethical conduct for research involving humans. Government of Canada; 2014: 1–220. http://www.pre.ethics.gc.ca/pdf/eng/tcps2-2014/TCPS_2_FINAL_Web.pdf [Google Scholar]
- 23. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4: 370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35: 1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 25. Glover EI, Phillips SM, Oates BR, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586: 6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipska KJ, Krumholz H, Soones T, Lee SJ. Polypharmacy in the aging patient: a review of glycemic control in older adults with type 2 diabetes. JAMA. 2016;315: 1034–1045. doi: 10.1001/jama.2016.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299: 1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 28. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53 Suppl 3: S16–S21. [DOI] [PubMed] [Google Scholar]
- 29. Henderson GC, Dhatariya K, Ford GC, et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23: 631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135: 681–686. [DOI] [PubMed] [Google Scholar]
- 31. Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012;303: E31–E39. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.