Abstract
Purpose of review
Almost half of all childhood deaths worldwide occur in children with malnutrition, predominantly in sub-Saharan Africa and South Asia. This review summarizes the mechanisms by which malnutrition and serious infections interact with each other and with children's environments.
Recent findings
It has become clear that whilst malnutrition results in increased incidence, severity and case fatality of common infections, risks continue beyond acute episodes resulting in significant postdischarge mortality. A well established concept of a ‘vicious-cycle’ between nutrition and infection has now evolving to encompass dysbiosis and pathogen colonization as precursors to infection; enteric dysfunction constituting malabsorption, dysregulation of nutrients and metabolism, inflammation and bacterial translocation. All of these interact with a child's diet and environment. Published trials aiming to break this cycle using antimicrobial prophylaxis or water, sanitation and hygiene interventions have not demonstrated public health benefit so far.
Summary
As further trials are planned, key gaps in knowledge can be filled by applying new tools to re-examine old questions relating to immune competence during and after infection events and changes in nutritional status; and how to characterize overt and subclinical infection, intestinal permeability to bacteria and the role of antimicrobial resistance using specific biomarkers.
Keywords: clinical trial, children, colonization, dysbiosis, environmental, growth, malnutrition, mortality, survival, susceptibility, undernutrition
INTRODUCTION
Worldwide, 5.6 million children die before their fifth birthday each year, with 80% of these deaths occurring in sub-Saharan Africa and Asia. Almost half of these deaths occur in children with malnutrition [1]. Strong epidemiological evidence suggests this is because of an elevated susceptibility to life-threatening infections amongst malnourished children. However, such studies do not disentangle the complex mechanisms underlying malnutrition, involving not only lack of nutrients, but also other risk factors such as exposure to pathogens, lack of access to healthcare and poverty. This review focusses on undernutrition among children in low and middle-income countries, with a focus on diarrhoea and pneumonia, the commonest childhood life-threatening infections worldwide. We discuss what is meant by the term ‘malnutrition’; how recent studies are informing our understanding of mechanisms linking anthropometric status and environment with susceptibility to life-threatening infections; and discuss implications and future research.
Box 1.
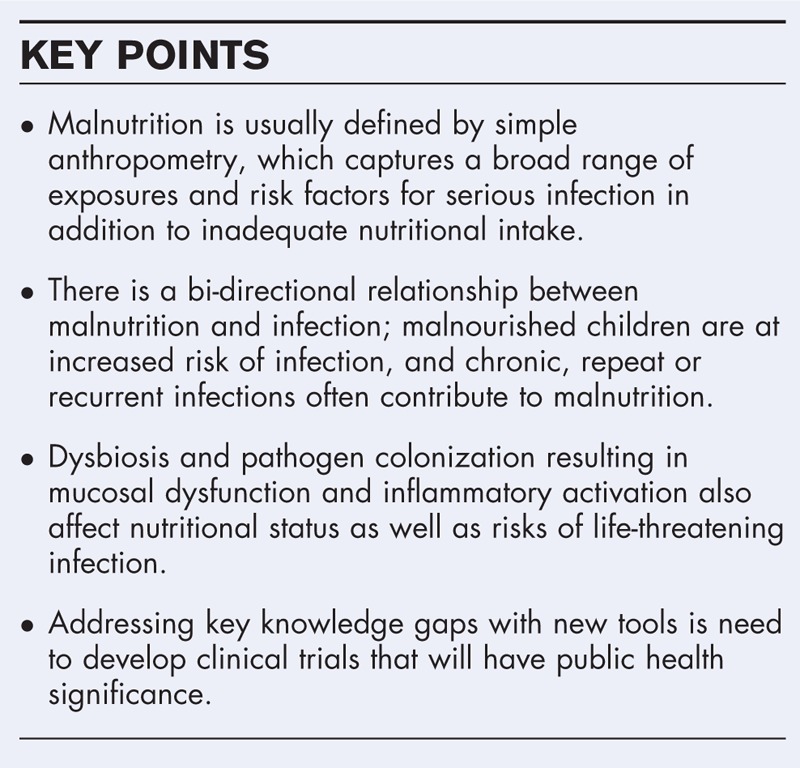
no caption available
WHAT IS MALNUTRITION?
The World Health Organization (WHO) defines malnutrition as deficiency, excess or imbalance in a person's intake of energy and/or specific nutrients in relation to their requirements [2].
Assessing malnutrition and risks of life-threatening infection
Energy and/or specific nutrient intake, requirements and expenditure are very rarely directly assessed. Instead, practice and research are based on anthropometric measures compared with a reference population. Wasting (thinness) is defined by weight-for-height/length (WHZ) among children under 5 years old, and BMI for age among 5–19-year olds. Stunting (linear growth impairment) is defined using height (or length)-for-age (HAZ).
Recently, there has been increased focus on the use of the mid-upper arm circumference (MUAC). MUAC is less affected by hydration status and generally it has better predictive value for subsequent mortality than WHZ [3,4]. However, cut offs to define malnutrition by MUAC based on its relationship with infectious disease or mortality outcomes had only been validated and used amongst children aged 6–59 months. Amongst infants (n = 2882) under 6 months old admitted to hospital in Kenya, MUAC was better at discriminating risk of subsequent inpatient death than WHZ [5]. A subset of these infants (n = 1405), were followed for 1 year after discharge; MUAC similarly had superior predictive value over WHZ. Similarly, amongst school-aged children and adolescents discharged from a rural hospital in Kenya (n = 1741) and amongst cohort of children over 5 years old with HIV infection in Uganda and Zimbabwe (n = 685), MUAC discriminated mortality risk at least as well as BMI-for-age [6]. This may result from MUAC directly measuring nutritional stores of protein (muscle) and fat whereas length is subject to significant measurement error among young infant.
Severe malnutrition can also be defined by the presence kwashiorkor, a syndrome characterized by nutritional oedema, often with skin depigmentation and sloughing, thinning of hair and inflammation. Why only some severely malnourished children develop kwashiorkor remains unknown [7]. Applications of new metabolomic, genomic and immunological techniques are addressing this question [8,9].
In addition to a diet low in energy or specific nutrients, a wide range of antenatal and postnatal environmental exposures, acute infection, chronic illness or psychosocial neglect may result in malnutrition [10]. It is clear from recent clinical trials enrolling children with severe malnutrition that they are also severely stunted (low height-for-age), suggesting chronic exposure to insult [11–13]. Hence, the commonly used term ‘severe acute malnutrition’ (SAM) has recently been challenged as a potentially misleading nomenclature with implications for successful intervention strategies [14▪]. We will use the term ‘malnutrition’ to mean low anthropometric values or kwashiorkor.
The interactions between episodic and chronic infections and malnutrition are complex and bi-directional. For example, children with malnutrition appear to be at substantially higher risk of diarrhoea, with both higher incidence and increased severity reported in malnourished children [15]. This risk appears to be correlated directly with degree of malnutrition as measured by anthropometry, with children with WAZ or HAZ 3 or less having a 37% increased risk of diarrhoea frequency and a 73% increase in average duration of diarrhoeal symptoms. At the same time, a meta-analysis assessing the impact of diarrhoea among several cohorts of children followed from birth until 24 months of age, demonstrated a 16% increase in stunting for every 5% increase in longitudinal incidence [16]. However, other studies have found mixed associations between frequent episodes of diarrhoea and long-term linear growth [17,18].
OUTCOMES OF INFECTION
In addition to an increased frequency of infectious disease, children with malnutrition are at significantly higher risk of more severe disease and suffer significantly more acute and long-term morbidity and mortality when infected. Recently, a clearer separation between the acute condition and background risks has been made.
Diarrhoea
Children with SAM are more likely to present to care with at least one integrated management of childhood illness (IMCI) danger sign and may be more likely to have a bacterial pathogen identified as a potential causative agent of their diarrhoea than nonmalnourished children [19▪]. In addition, as demonstrated in a study of 1146 children admitted to hospital with moderate-severe diarrhoea in Western Kenya (2005–2007), among children with severe acute malnutrition, risk of death following an episode of diarrhoea was four times higher than better nourished children [20]. The community-based Global Enteric Multicenter Study (GEMS) also enrolled 9439 children with moderate-to-severe diarrhoea and control children without diarrhoea in seven countries in Africa and Asia [21]. Diarrhoea case status was associated with stunting (chronic malnutrition leading to linear growth failure) as was postdiarrhoea mortality during 90 days, for which each z score unit of HAZ was associated with a reduction in the risk of death by 26–53% depending on age.
Pneumonia
Similarly, malnutrition is not only associated with an increased risk of pneumonia episodes, but increased severity and case fatality. Development of an inpatient paediatric pneumonia mortality risk score (RISC) in Malawi (n = 16 475) [22,23], identified severe malnutrition as having similar predictive value to hypoxaemia and coma [21]. In Kenya, among 4187 children admitted to hospital with severe pneumonia, 25% were severely malnourished, again a strong risk factor for inpatient death alongside signs of disease severity [24▪]. A subset of children was followed after discharge from hospital; 37% of deaths occurred after discharge. Malnutrition, young age, HIV status and prolonged hospital admission were associated with postdischarge mortality, whilst pneumonia severity indicators were not, suggesting that an episode of severe pneumonia is a marker of background risk.
MECHANISMS
A classic monograph by Scrimshaw in 1968, ‘Interactions of Nutrition and Infection,’ sets out a vicious cycle between nutrition and infection [25]. He proposed that malnutrition resulted in infections, infections resulted in malnutrition by anorexia, malabsorption, and diversion, loss, and increased requirements of nutrients. Currently, a more nuanced understanding is emerging of the roles of the environment, burden of exposure to pathogens because of crowding or poor water and sanitation, gut microbiota, chronic intestinal inflammation, mucosal barrier loss and immune function (Fig. 1).
FIGURE 1.
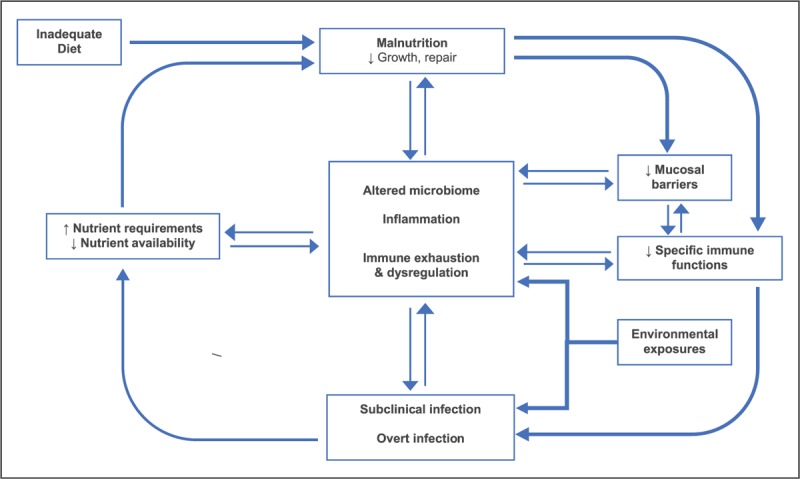
A growing understanding of a ‘vicious cycle’: interactions between malnutrition, infection and intestinal dysfunction.
Dysbiosis and mucosal integrity
A key concept in understanding this relationship is that colonization of gut, respiratory and other mucosal surfaces, is a precursor to invasive infection. Malnutrition is typically accompanied by dysbiosis (change in the normal pattern of colonizing organisms) and disturbances in normal barrier functions.
The environment of the intestine plays a critical role as the main interface between the child and the nutrients and energy required to sustain growth. In addition to the critical function of modulating absorption and secretion, the enteric system is the predominant lymphoid tissue in the body. The surface of the gut functions as a major site of pathogen recognition and response, and is a critical barrier to pathogen translocation. Finally, the enteric system is a key site of hormonal modulation, regulating key functions related to metabolism and growth.
Although diarrhoea is a common manifestation of enteric infection and dysbiosis within the gut, many children experience significant intestinal dysfunction even in the absence of overt diarrheal disease. In many settings, chronic exposure to faecally contaminated environments may lead to an asymptomatic syndrome of poor absorption, local intestinal inflammation and increased translocation of bacterial products across the gut surface (environmental enteric dysfunction (EED) [26]. In many settings, markers associated with EED can be detected in as many as half of all children and these markers have been strongly associated with future linear growth failure [27,28].
EED can be identified by the presence of crypt atrophy and villous hyperplasia in the small intestine. In addition, a number of markers of intestinal permeability, absorption, inflammation and intestinal repair have been associated with the presence of EED [18,26,29]. These abnormalities lead to impaired absorptive capacity, local inflammation and disruption of tight junctions. This increases the potential for translocation of bacterial products and systemic immune activation. Although much attention has been paid to the local intestinal effects of EED on permeability, absorption and inflammation, the increased systemic inflammation and activation seen in malnourished children may be more important in explaining associations between malnutrition, gut dysfunction, long-term morbidity and mortality. In Malawi, both intestinal and systemic inflammation were associated with mortality risk in severely acute malnourished children and that this was not mediated by the presence of specific intestinal pathogens [30▪].
Similarly, malnutrition is associated with small intestinal histological abnormalities, including villous blunting, reduction in mucus-secreting goblet cells and inflammation [31]. It is not yet clear if this is EED or a separate disease. Previous metagenomic studies have suggested reduced microbial diversity in relation to a child's age and an increase in potent pathogenic Enterobacteriaceae in malnourished children [32]. In addition, several seminal studies have demonstrated growth failure in mice after receiving transplanted microbiota from malnourished children [33]. Increasing evidence suggests that the microbiome, both measured by diversity and taxa distribution, is a critical modulator of homeostasis within the gut, influencing absorption, immune function and hormonal regulation [34].
In addition to the general microbial milieu of the gut, specific pathogens have also been identified as potential mediators or drivers of malnutrition in some settings. In an urban community in Dhaka, Bangladesh, malnourished children (n = 486) and well nourished controls (n = 442) were investigated for a wide range of enteropathogens by Taqman Array Card. The presence of enteroaggregative Escherichia coli, heat-labile toxin producing E. coli, Shigella/enteroinvasive E. coli, Campylobacter spp., norovirus genogroup 1, and Giardia spp. were associated with malnutrition [35▪]. The number of different pathogens detected was inversely associated with subsequent growth, indicating clinically significant dysbiosis. This was further explored among 1684 children across eight sites in South Asia, Africa and Latin American by the Mal-ED study group [36▪]. Intestinal inflammation and growth were associated with the presence of enteroaggregative E. coli.
There are parallels at respiratory mucosal surfaces. In Ethiopia, nasopharyngeal carriage of Streptococcus pneumonia was assessed in 361 children at an outpatient clinic [37]. Overall, 44% were colonized by S. pneumoniae (not serotyped, 18% multidrug resistant) and colonization was associated with the number of siblings in the household and presence of malnutrition defined by weight-for-age, capturing aspects of both wasting and stunting: adjusted odds ratio 2.1 [95% confidence interval (CI) 1.2–3.4]. In Venezuela, amongst 1064 children living in rural areas of the Orinoco Delta, S. pneumoniae colonization was (nonsignificantly on multivariable analysis) more common among stunted children who were stunted [38]. However, a significant association had been previously shown in another population in Venezuela, with a 33% reduction in colonization per unit HAZ, and clear association between colonization and acute respiratory infection [39]. Pneumococcal colonization was not reported in relation to malnutrition in the multicentre PERCH study at nine sites in seven countries [40].
Pathogens
Clinical outcomes in children with malnutrition might differ if they are infected with different organisms causing the same clinical syndrome (e.g. bacterial rather than viral cause), or if they present with the same pathogen but have an increased risk of antimicrobial resistance (AMR). The latter might occur with increased exposure to healthcare and antimicrobials or reduced pathogen clearance. In addition, children with malnutrition may simply respond differently to pathogen challenge, a range of abnormalities across multiple pathways in the innate and adaptive immune system have been described in these children [41,42]. Recent studies that standardize clinical conditions and their causative organisms are especially informative to our understanding.
Studies have demonstrated variable associations between malnutrition and bacteraemia risk. Some previous studies have suggested an increased likelihood of Gram negative bacteraemia in malnourished children [43]. However, the range of bacterial species is typically similar to those observed in nonmalnourished children in low-resource settings. One recent blood culture study from Tanzania reported a high prevalence of Pseudomonas spp. (36%)., Enterobacter spp. (16%), and Staphylococus aureus (15%), suggesting limited sensitivity to first line ampicillin with gentamicin. It is unclear if these were community-acquired (at admission) isolates or hospital-acquired (after admission) isolates. In Kenya, contrary to previous case series suggesting that coagulase-negative staphylococci (CONS, usually associated with invasive medical devices) may be important pathogens in severely malnourished children, there was no association between CONS being identified on blood culture and mortality or duration of hospitalization [44]. Further data is urgently needed on AMR in this context.
Vaccine efficacy
Early studies of vaccine responses among malnourished children suggesting reduced efficacy of oral vaccines (polio, rotavirus), but no differences in antibody titres following parenterally given vaccines [45,46]. However, true efficacy or effectiveness against pathogen challenge remained uncertain. In a landmark South African case–control study, receipt of two or more doses of 13-valent pneumococcal conjugate vaccine was demonstrated to be as effective (90%) in preventing proven invasive pneumococcal disease among malnourished children as in well nourished children [47▪▪]. Thus, whilst altered vaccine efficacy may impact rotavirus disease in relation to malnutrition, it does not explain an increased susceptibility to common respiratory pathogens.
CLINICAL TRIALS
Clinical trials have attempted to interrupt the cycle described above. In Kenya and Bangladesh, large-scale combined water, sanitation hygiene and nutrition interventions had minimal effects on diarrhoea or growth. In a multicentre trial in Kenya, long-term prophylaxis daily co-trimoxazole did not reduce postdischarge serious infections or improve growth during 1 year among severely malnourished children [12]. Trials of other antimicrobials to treat complicated severe malnutrition and/or prevent postdischarge mortality targeting dysbiosis and small intestinal bacterial overgrowth are underway [48]. Preventive trials using candidate probiotic organisms and prebiotic foods are also in progress as microbiota are better characterized [9,32]. Future trials may also involve systemic or gut-specific immunomodulation.
FUTURE PERSPECTIVES
Despite a reduction in overall child mortality in the last 25 years, it is clear from recent modelling that most of Africa is highly unlikely to achieve the Sustainable Development Goal target of ending malnutrition by 2030 [49]. The Child Health and Mortality Prevention Surveillance (CHAMPS) project (https://champshealth.org) aims to determine causes of death in resource-poor populations through minimally invasive postmortem tissue sampling, which may better target therapy in life. The Childhood Acute Illness and Nutrition (CHAIN) Network (http://chainnetwork.org) aims to identify modifiable biomedical and socioeconomic risks to take forward in clinical trials.
CONCLUSION
The malnutrition–environment–infection axis is complex and not easily addressed by individual interventions. Better understanding will come through applying new tools to re-examine longitudinal immune competence ex vivo in relation to infection events and changes in nutritional status, more specific biomarkers of infection, correlates of intestinal function and bacterial translocation, microbial populations and causes of disease.
Acknowledgements
None.
Financial support and sponsorship
The authors were supported by the Bill & Melinda Gates Foundation (grant number OPP1131320). J.A.B. is supported by the MRC/DfID/Wellcome Trust Global Health Trials Scheme (grant number MR/M007367/1).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Black RE, Victora CG, Walker SP, et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382:427–451. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation. What is malnutrition? Available at: http://www.who.int/features/qa/malnutrition/en/. [Google Scholar]
- 3.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull 2006; 27 (3 Suppl):S7–S23. [DOI] [PubMed] [Google Scholar]
- 4.Briend A, Alvarez J-L, Avril N, et al. Low mid-upper arm circumference identifies children with a high risk of death who should be the priority target for treatment. BMC Nutr 2016; 2:63. [Google Scholar]
- 5.Mwangome M, Ngari M, Fegan G, et al. Diagnostic criteria for severe acute malnutrition among infants aged under 6 mo. Am J Clin Nutr 2017; 105:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mramba L, Ngari M, Mwangome M, et al. A growth reference for mid upper arm circumference for age among school age children and adolescents, and validation for mortality: growth curve construction and longitudinal cohort study. BMJ 2017; 358:j3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heikens GT, Manary M. 75 years of Kwashiorkor in Africa. Malawi Med J 2009; 21:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Giovanni V, Bourdon C, Wang DX, et al. Metabolomic changes in serum of children with different clinical diagnoses of malnutrition. J Nutr 2016; 146:2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tidjani Alou M, Million M, Traore SI, et al. Gut bacteria missing in severe acute malnutrition, can we identify potential probiotics by culturomics? Front Microbiol 2017; 8:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzaroni S, Wagner N. Misfortunes never come singly: structural change, multiple shocks and child malnutrition in rural Senegal. Econ Hum Biol 2016; 23:246–262. [DOI] [PubMed] [Google Scholar]
- 11.Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013; 368:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isanaka S, Langendorf C, Berthe F, et al. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 2016; 374:444–453. [DOI] [PubMed] [Google Scholar]
- 13.Berkley JA, Ngari M, Thitiri J, et al. Daily co-trimoxazole prophylaxis to prevent mortality amongst children with complicated severe acute malnutrition: a randomised, double-blind, placebo controlled trial. Lancet Glob Health 2016; 4:e464–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Bhutta ZA, Berkley JA, Bandsma RHJ, et al. Severe childhood malnutrition. Nat Rev Dis Primers 2017; 3:17067. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review of the pathophysiology and treatment of severe malnutrition.
- 15.Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg 1992; 47 (1 Pt 2):28–35. [DOI] [PubMed] [Google Scholar]
- 16.Checkley W, Buckley G, Gilman RH, et al. Multicountry analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008; 37:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard SA, Black RE, Gilman RH, et al. Diarrhea in early childhood: short-term association with weight and long-term association with length. Am J Epidemiol 2013; 178:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper KM, Mutasa M, Prendergast AJ, et al. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis 2018; 12:e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Tickell KD, Pavlinac PB, John-Stewart GC, et al. Impact of childhood nutritional status on pathogen prevalence and severity of acute diarrhea. Am J Trop Med Hyg 2017; 97:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the effect of being wasted on clinical danger signs in childhood diarrhea and detection of enteroaggregative Escherichia coli. This illustrates differences in pathogens causing a more severe presentation of the same clinical syndrome among malnourished children.
- 20.O’Reilly CE, Jaron P, Ochieng B, et al. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005-2007: a cohort study. PLoS Med 2012; 9:e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–222. [DOI] [PubMed] [Google Scholar]
- 22.Hooli S, Colbourn T, Lufesi N, et al. Correction: predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS One 2018; 13:e0193557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooli S, Colbourn T, Lufesi N, et al. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS One 2016; 11:e0168126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪.Ngari MM, Fegan G, Mwangome MK, et al. Mortality after inpatient treatment for severe pneumonia in children: a cohort study. Paediatr Perinat Epidemiol 2017; 31:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the effect of malnutrition, independent of HIV status, on both inpatient and postdischarge mortality. Disease severity, HIV and nutritional status had a differential effect before and after discharge.
- 25.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 2003; 133:316S–321S. [DOI] [PubMed] [Google Scholar]
- 26.Denno DM, Tarr PI, Nataro JP. Environmental enteric dysfunction: a case definition for intervention trials. Am J Trop Med Hyg 2017; 97:1643–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosek M, Haque R, Lima A, et al. MAL-ED network. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 2013; 88:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naylor C, Lu M, Haque R, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015; 2:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arndt MB, Richardson BA, Ahmed T, et al. Fecal markers of environmental enteropathy and subsequent growth in bangladeshi children. Am J Trop Med Hyg 2016; 95:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪.Attia S, Versloot CJ, Voskuijl W, et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: an observational cohort study. Am J Clin Nutr 2016; 104:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study in Malawi, diarrhea, intestinal inflammation, low concentrations of fecal short-chain fatty acids, and systemic inflammation were associated with mortality among malnourished children, irrespective of fecal pathogens detected. It demonstrates system-wide effects, rather than effects only mediated by intestinal infection.
- 31.Attia S, Feenstra M, Swain N, et al. Starved Guts: Morphologic and Functional Intestinal Changes in Malnutrition. J Pediatr Gastroenterol Nutr 2017; 65:491–495. [DOI] [PubMed] [Google Scholar]
- 32.Million M, Diallo A, Raoult D. Gut microbiota and malnutrition. Microb Pathog 2017; 106:127–138. [DOI] [PubMed] [Google Scholar]
- 33.Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013; 339:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tickell KD, Walson JL. Nutritional enteric failure: neglected tropical diseases and childhood stunting. PLoS Negl Trop Dis 2016; 10:e0004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Platts-Mills JA, Taniuchi M, Uddin MJ, et al. Association between enteropathogens and malnutrition in children aged 6-23 mo in Bangladesh: a case-control study. Am J Clin Nutr 2017; 105:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrates a relationship between enteric pathogens and subsequent nutritional status among malnourished children living in the community in urban Bangladesh. Pathogen control is discussed as part of malnutrition prevention.
- 36▪.Lima AAM, Soares AM, Filho JQS, et al. Enteroaggregative Escherichia coli subclinical infection and coinfections and impaired child growth in the MAL-ED Cohort Study. J Pediatr Gastroenterol Nutr 2018; 66:325–333. [DOI] [PubMed] [Google Scholar]; At eight sites in low and middle-income countries, this report highlights the importance of intestinal enteroaggregative Escherichia coli's effect on growth depending on co-infection. The role of sub-clinical infections is discussed.
- 37.Gebre T, Tadesse M, Aragaw D, et al. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in Southwest Ethiopia. Children (Basel) 2017; 4: pii: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhagen LM, Hermsen M, Rivera-Olivero IA, et al. Nasopharyngeal carriage of respiratory pathogens in Warao Amerindians: significant relationship with stunting. Trop Med Int Health 2017; 22:407–414. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen LM, Gomez-Castellano K, Snelders E, et al. Respiratory infections in Enepa Amerindians are related to malnutrition and Streptococcus pneumoniae carriage. J Infect 2013; 67:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggett HC, Watson NL, Deloria Knoll M, et al. PERCH Study Group. Density of upper respiratory colonization with Streptococcus pneumoniae and its role in the diagnosis of Pneumococcal Pneumonia among children aged <5 years in the PERCH Study. Clin Infect Dis 2017; 64:S317–S327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rytter MJ, Kolte L, Briend A, et al. The immune system in children with malnutrition–a systematic review. PLoS One 2014; 9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourke CD, Berkley JA, Prendergast AJ. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol 2016; 37:386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39–47. [DOI] [PubMed] [Google Scholar]
- 44.Obiero CW, Seale AC, Jones K, et al. Should first-line empiric treatment strategies cover coagulase-negative staphylococcal infections in severely malnourished or HIV-infected children in Kenya? PLoS One 2017; 12:e0182354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savy M, Edmond K, Fine PE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr 2009; 139:2154s–2218s. [DOI] [PubMed] [Google Scholar]
- 46.Parker EP, Ramani S, Lopman BA, et al. Grassly NC: causes of impaired oral vaccine efficacy in developing countries. Future Microbiol 2018; 13:97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪▪.Cohen C, von Mollendorf C, de Gouveia L, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South African children: a case-control study. Lancet Glob Health 2017; 5:e359–e369. [DOI] [PubMed] [Google Scholar]; This important study reveals that receipt of two or more doses of pneuococcal conjugate vaccine was unaffected by nutritional status among HIV-uninfected children. Despite previous studies of antibody responses, actual efficacy had not previously been evaluated.
- 48.First line antimicrobials in children with complicated severe acute malnutrition (FLACSAM). Available at: https://clinicaltrials.gov/ct2/show/NCT03174236. [Google Scholar]
- 49.Osgood-Zimmerman A, Millear AI, Stubbs RW, et al. Mapping child growth failure in Africa between 2000 and 2015. Nature 2018; 555:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


