The American College of Physicians (ACP) recently issued guidelines for the treatment of low bone density or osteoporosis to prevent fractures [1]. ACP recommends that clinicians offer pharmacological treatment to reduce spine and hip fracture risk in women with osteoporosis, and consider treatment in women at high risk. More specifically, ACP strongly recommends ‘that clinicians offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk for hip and vertebral fractures in women who have known osteoporosis’.
The recommendations are largely based on a systematic review of randomized controlled trials. Whereas a review of such evidence is to be commended, the guideline largely ignores other important evidence that might modulate the way guidelines are formulated [2]. The areas of concern discussed below, include the scope of guidelines, the position on FRAX, and the use of anabolic interventions.
Scope
The restricted scope of the ACP guidelines is exemplified in the title that refers to treatment in men and women with osteoporosis. Two definitions of osteoporosis are provided; one, the WHO definition based on the T-score for bone mineral density and the other an individual with a prior fragility fracture. Except for teriparatide (not recommended as a first line treatment), the ACP guideline considers that the evidence is insufficient to recommend treatment of patients with prior fracture with other interventions. Thus, the gateway to treatment is a BMD diagnosis of osteoporosis though no recommendations are provided on who should have a BMD measurement in the first place. The guideline fails in the sense that there is no indication to whom they apply and no reference to any relevant literature.
The use of BMD as the exclusive gateway to assessment is problematic for many reasons. Problems include the low sensitivity of BMD for fracture prediction [3, 4], the different significance of a given T-score according to age [5] and risks that differ according to latitude [6] socioeconomic prosperity [7] and country [8]. In many countries, intervention thresholds, historically based on the T-score for BMD, have been replaced in recent years by more complete risk assessment tools that take account of the added weight of the risk factors on fracture risk [9–13]. For example, twenty years ago, the European Foundation for Osteoporosis (now the International Osteoporosis Foundation) issued guidelines for the diagnosis and management of osteoporosis [14], shortly followed by those of the Royal College of Physicians in the UK [15]. Both organisations recommended treatment in patients with osteoporosis based on bone mineral density measurements. Prospective patients to be referred for bone densitometry were identified by the presence of clinical risk factors associated with osteoporosis. The guidance utilised risk factors for fracture as an initial step for assessment, but recommended treatment only in individuals with a T-score of -2.5. Guidelines in the UK [16, 17 and Europe [18, 19] and many other] countries [20] now accommodate FRAX as the primary gateway to risk assessment. Against this shift, the ACP updated guidance, firmly entrenching a T-score treatment threshold of -2.5 SD, is reminiscent of European and UK guidance some 20 years ago [14, 15].
Review of FRAX
The ACP guidance eschews the use of FRAX and states that there is no evidence from randomised control trials demonstrating a benefit of fracture reduction when FRAX scores are used for treatment decision making. The argument implies that the beneficial effects of treatment on fracture risk is restricted to patients with osteoporosis. Irrespective of the veracity of the statement, the argument presupposes that high FRAX scores with or without BMD do not identify individuals with low bone mineral density—a supposition that has for several years been shown to be ill-founded [21–23]. An example of the application of the guidelines used by the National Osteoporosis Guideline Group (NOGG) in the UK is given in Table 1, which shows that the case-finding strategy identifies women with low BMD. Moreover, the conclusion that these drugs only act in BMD proven osteoporosis is a flawed one, driven by subgroup analyses, most of which are post hoc [24]. Indeed, the relevant question in a more statistically appropriate manner is; is there an interaction between the effect of treatment and baseline BMD? All studies that have examined this for the outcome of vertebral fractures have not shown any impact of baseline BMD on risk reduction during therapy.
Table 1.
NOGG strategy applied to women from without prior fracture, by age (/1000) [21] with kind permission from Springer Science+Business Media B.V.
| Age (years) | Number scanned | Number selected | Expected hip fractures | Expected MOF | Mean FN T-score |
|---|---|---|---|---|---|
| 50 | 63 | 22 | 2 | 52 | -1.78 |
| 55 | 48 | 16 | 2 | 27 | -2.28 |
| 60 | 59 | 14 | 2 | 17 | -2.67 |
| 65 | 131 | 38 | 7 | 48 | -2.58 |
| 70 | 140 | 29 | 8 | 38 | -2.91 |
| 75 | 89 | 18 | 6 | 23 | -3.35 |
| 80 | 69 | 15 | 6 | 16 | -3.60 |
| 85 | 50 | 15 | 7 | 20 | -3.66 |
| 40 | 241 |
FN femoral neck
MOF major osteoporotic fracture (hip, clinical spine, forearm, proximal humerus)
The ACP acknowledges that ‘moderate-quality evidence from post hoc analysis of 1 RCT showed no significant interaction between fracture risk as assessed by FRAX and the efficacy of raloxifene for reducing the relative risk for vertebral fractures in women older than 75 years. It neglected to indicate that similar findings for a majority of interventions used in osteoporosis including strontium ranelate, [25], raloxifene [26, 27], bazedoxifene [26], clodronate [28], daily and weekly teriparatide [29, 30], abaloparatide [31], denosumab [32] alendronate [33] as well as a basket of interventions used by general practitioners in the UK [34]. Most of these were post hoc but, in the case of denosumab, was a pre-planned analysis. In addition, the ‘screening for prevention of fractures in older women’ (SCOOP) study was a prospective randomised study that demonstrated efficacy for hip fracture in women selected on the basis of hip fracture probability assessed using FRAX [34].
It is axiomatic that different intervention thresholds will identify different patients at different risk. Some examples are given in Table 2 based on the National Health and Nutrition Examination Survey (NHANES) 2005–2008 [35, 36]. It is of interest that the ACP guideline selects the greater number of patients eligible for treatment than an age-specific FRAX threshold but the latter identifies a population at much higher risk.
Table 2.
Number selected as being above the intervention threshold and the proportion who will fracture over 10 years (mean 10-year fracture probability of major osteoporotic fracture (MOP) and hip fracture) in men and women aged 50 years or more from the NHANES cohort according to different intervention thresholds [36, with kind permission of John Wiley and Sons].
| men | women | |||||
|---|---|---|---|---|---|---|
| % who fracture | % who fracture | |||||
| Selection | N | MOP | Hip | N | MOP | Hip |
| None | 1959 | 6.0 | 1.5 | 1649 | 10.2 | 2.4 |
| FRAX fixed thresholdsa | 266 | 13.5 | 6.3 | 387 | 21.2 | 7.9 |
| FRAX at fracture thresholdb | 54 | 16.3 | 4.0 | 144 | 26.0 | 9.7 |
| FRAX fixed thresholds + prior fracturec | 326 | 12.3 | 5.3 | 414 | 20.5 | 7.5 |
| FRAX at fracture threshold + prior fracturec | 121 | 11.9 | 2.9 | 179 | 23.4 | 8.2 |
| NOFd | 330 | 11.7 | 4.9 | 511 | 17.7 | 6.2 |
| Prior fracturec | 71 | 8.9 | 2.1 | 57 | 19.0 | 6.1 |
| T-score ≤-2.5e | 79 | 11.2 | 5.4 | 298 | 17.3 | 6.7 |
| Prior fracture & T-score ≤-2.5e | 148 | 9.9 | 3.6 | 335 | 17.0 | 6.4 |
FRAX with 20% and 3% probability thresholds for major fracture and hip fracture respectively
FRAX with age-specific thresholds plus prior fracture
Prior hip or spine fracture
National Osteoporosis Foundation Guidelines [37]
T-score at proximal femur or lumbar spine
Missed opportunity for anabolic treatment
The ACP guideline recommends that clinicians offer pharmacologic treatment with alendronate, risedronate, zoledronic acid, or denosumab to reduce the risk for hip and vertebral fractures in women who have known osteoporosis. These drugs strengthen trabecular bone by reducing bone turnover but do not rebuild the damaged trabecular architecture. They have less or even no effect on strengthening cortical bone. Perhaps the greatest deficit of the ACP guideline is the dismissive attitude to anabolic treatment such as teriparatide, parathyroid hormone and abaloparatide. The guideline acknowledges that treatment with teriparatide reduces radiographic vertebral and nonvertebral fractures compared with placebo in postmenopausal osteoporotic women. The position is predicated by the view of the ACP that there are no differences in efficacy between available interventions.
Gains in BMD following treatment with teriparatide and abaloparatide are very substantial compared with the bisphosphonates, SERMs and denosumab and are expected to be translated in terms of fracture risk reduction. Indeed, in a head to head comparative trial, teriparatide has been shown to have superior efficacy than risedronate on vertebral and clinical fractures [38]. The relative risk reduction for clinical vertebral fractures was 56 % (95% CI=32-71 %) and that for all clinical fractures 52 % (26- 68 %). In a head-to-head comparison, abaloparatide increased BMD and reduced the risk of non-vertebral fractures more than teriparatide and, indeed, more rapidly [39]. Romosozumab (still an investigational agent) followed after 12 months by alendronate for a further year showed superior efficacy on fracture outcomes compared with alendronate alone, albeit with some concerns over adverse cardiovascular effects [40]. These observations indicate the emergence of a rank order of efficacy unappreciated by the ACP.
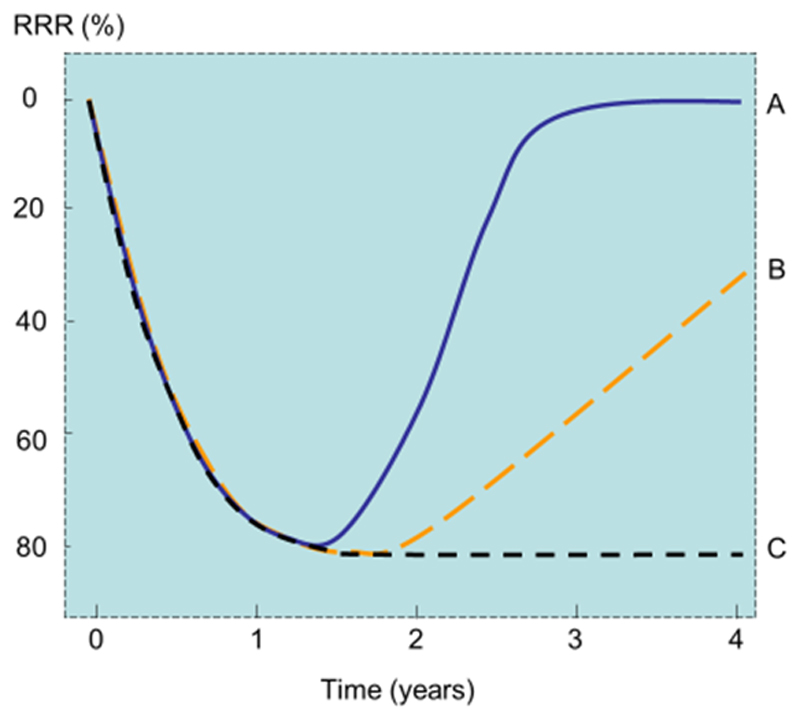
Given that treatments with anabolic agents are limited to 18-24 months and given that efficacy will wane once treatment is stopped, the real potential of the anabolic treatments is whether their greater effect on BMD and fracture can be maintained with the inhibitors of bone turnover once treatment is stopped (Figure 1) [41]. In the case of abaloparatide, efficacy was shown in an 18-month placebo control study with significant reductions in risk of vertebral, nonvertebral, clinical, and major osteoporotic fractures compared with placebo [39]. Efficacy was maintained with a subsequent 24-month treatment with alendronate (70 mg weekly) given to patients previously taking or abaloparatide or placebo (scenarios A and B in Figure 1). The relative risk reduction for vertebral fracture seen in the placebo control phase of the study (RRR=86 %) was maintained in the extension phase of the trial (RRR=84 %). A sustained effect was also seen for non-vertebral fracture, major osteoporotic fracture and hip fracture risk reduction [42].
Fig. 1.
Schematic diagram showing the effects of a bone-forming agent on the relative fracture risk reduction (RRR) compared with placebo. In scenario A, treatment with a bone-forming agent induces a marked effect on fracture risk over an 18 months exposure compared with placebo. On stopping the agent, the effect on fracture wears off over a similar time interval of 18 months. In scenario C, the treatment group is treated after the exposure with an inhibitor of bone turnover which maintains the efficacy up to 4 years. In scenario B, both the treatment and the placebo groups are treated after the exposure with an inhibitor of bone turnover [adapted from 41].
The advent of anabolic therapy that can be sustained past the duration of exposure, opens a new era in the management of osteoporosis, particularly those patients at imminent risk [43].
Conclusion
We recognize that several papers in this editorial post-date the review of the ACP though the sentiments were well rehearsed. Notwithstanding, the ACP guideline appears outdated even at its time of publication.
Acknowledgements
Committee of Scientific Advisors and the Committee of National Societies of the International Osteoporosis Foundation (to complete)
Footnotes
Competing Interests
JAK reports grants from Amgen, Eli Lilly and Radius Health; non-financial support from Medimaps, and Asahi; and other support from AgNovos. JAK is the architect of FRAX but has no financial interest. Professor Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. RR has received consulting fees or advisory board fees from Radius Health, Labatec, Danone, and Nestlé. J-YR has received advisory board or speaker fees from Asahi-Kasei, Eli Lilly, IBSA- Genévrier, Nycomed-Takeda, PharmEvo, Radius Health, Roche, Servier, UCB, Will Pharma and Zodiac.
References
- 1.Qaseem A, Forciea MA, McLean RM, Denberg TD, Clinical Guidelines Committee of the American College of Physicians Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818–839. doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 2.Caplan L, Hansen KE, Saag KG. Response to the ACP osteoporosis guideline 2017 Revision. Arthritis Rheumatol. 2017 doi: 10.1002/art.40305. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575–580. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation. WHO Technical Report Series. Vol. 843. WHO; Geneva: 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. [PubMed] [Google Scholar]
- 5.Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to World Health Organization criteria for osteoporosis and osteopenia. Bone. 2000;27:585–590. doi: 10.1016/s8756-3282(00)00381-1. [DOI] [PubMed] [Google Scholar]
- 6.Odén A, Kanis JA, McCloskey EV, Johansson H. The effect of latitude on the risk and seasonal variation in hip fracture in Sweden. J Bone Miner Res. 2014;29:2217–2223. doi: 10.1002/jbmr.2250. [DOI] [PubMed] [Google Scholar]
- 7.Johnell O, Borgstrom F, Jonsson B, Kanis J. Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporosis International. 2007;18:333–337. doi: 10.1007/s00198-006-0245-4. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cooper Cyrus, on behalf of the IOF Working Group on Epidemiology and Quality of Life A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–1444. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFracture Scores. BMJ. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dachverband Osteologie e.V. DVO guideline 2009 for prevention, diagnosis and therapy of osteoporosis in adults. Osteologie. 2011;20:55–74. [Google Scholar]
- 12.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanis JA, on behalf of the World Health Organization Scientific Group . Technical Report. WHO Collaborating Centre, University of Sheffield; UK: 2008. Assessment of osteoporosis at the primary health-care level. Available at http://www.shef.ac.uk/FRAX/index.htm. [Google Scholar]
- 14.Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D, on behalf of the EFFO Guidelines for diagnosis and management of osteoporosis. Osteoporosis International. 1997;7:390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- 15.Royal College of Physicians. Osteoporosis: Clinical guidelines for prevention and treatment. Royal College of Physicians; London: 1999. [Google Scholar]
- 16.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, et al. The National Osteoporosis Guideline Group (NOGG) UK clinical guideline for the prevention and treatment of osteoporosis. Archives of Osteoporosis. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compston J, Cooper A, Cooper C, et al. on behalf of the National Osteoporosis Guideline Group (NOGG) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009;62:105–108. doi: 10.1016/j.maturitas.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F, et al. for the Joint IOF-ECTS GIO Guidelines Working Group A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23:2257–76. doi: 10.1007/s00198-012-1958-1. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Harvey NC, Cyrus Cooper C, Johansson H, Odén A, McCloskey EV, the Advisory Board of the National Osteoporosis Guideline Group A systematic review of intervention thresholds based on FRAX. A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson H, Kanis JA, Oden A, Compston J, McCloskey E. A comparison of case-finding strategies in the UK for the management of hip fractures. Osteoporos Int. 2012;23:907–915. doi: 10.1007/s00198-011-1864-y. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAXs with and without BMD. Calcif Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 23.Leslie WD, Majumdar SR, Lix L, Johansson H, McCloskey EV, Kanis JA. High fracture probability with FRAX usually indicates densitometric osteoporosis: implications for clinical practice. Osteoporos Int. 2012;23:391–397. doi: 10.1007/s00198-011-1592-3. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey E. A BMD threshold for treatment efficacy in osteoporosis? A need to consider the whole evidence base. Osteoporos Int. 2016;27:417–9. doi: 10.1007/s00198-015-3406-5. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA, Jönsson B, Odén A, McCloskey EV. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX®. Osteoporos Int. 2011;22:2347–2355. doi: 10.1007/s00198-010-1474-0. with erratum Osteoporos Int 22: 2357-2358. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA, Johansson H, Oden A, McCloskey EV. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX®. Bone. 2009;44:1049–54. doi: 10.1016/j.bone.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Kanis JA, Johansson H, Oden A, McCloskey EV. A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX®. Bone. 2010;47:729–735. doi: 10.1016/j.bone.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 28.McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, et al. Ten-year fracture probability identifies women who will benefit from clodronate therapy--additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int. 2009;20:811–817. doi: 10.1007/s00198-008-0786-9. [DOI] [PubMed] [Google Scholar]
- 29.Harvey NC, Kanis JA, Odén A, Burge RT, Mitlak BH, Johansson H, McCloskey EV. FRAX and the effect of teriparatide on vertebral and non-vertebral fracture. Osteoporos Int. 2015a;26:2347–53. doi: 10.1007/s00198-015-3173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey NC, Kanis JA, Odén A, Nakamura T, Shiraki M, Sugimoto T, Kuroda T, Johansson H, McCloskey EV. Efficacy of weekly teriparatide does not vary by baseline fracture probability calculated using FRAX. Osteoporos Int. 2015b;26:2347–2354. doi: 10.1007/s00198-015-3129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCloskey EV, Johansson H, Oden A, Harvey NC, Jiang H, Modin S, et al. The effect of abaloparatide-SC on fracture risk is independent of baseline FRAX fracture probability: A post hoc analysis of the ACTIVE Study. J Bone Miner Res. 2017;32:1625–1631. doi: 10.1002/jbmr.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, et al. Denosumab reduces the risk of all osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX®. J Bone Miner Res. 2012;27:1480–1486. doi: 10.1002/jbmr.1606. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR. Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: The Fracture Intervention Trial. J Bone Miner Res. 2012;27:1804–10. doi: 10.1002/jbmr.1625. [DOI] [PubMed] [Google Scholar]
- 34.Shepstone L, Lenaghan E, Cooper C, Clarke S, Fordham R, Gittoes NJ, Harvey IM, Harvey NC, Heawood A, Holland R, Howe A, et al. A randomized controlled trial of screening in the community to reduce fractures in older women – The SCOOP Study. Lancet. 2017 doi: 10.1016/S0140-6736(17)32640-5. in press. [DOI] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B, Looker AC, Tosteson ANA, Johansson H, Kanis JA, Melton LJ. The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the U.S.: an update in NHANES 2005-2008. Osteoporos Int. 2012;23:811–820. doi: 10.1007/s00198-011-1694-y. [DOI] [PubMed] [Google Scholar]
- 36.Kanis JA, McCloskey EV, Harvey NC, Johansson H, Leslie WD. Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res. 2015;30:1747–1753. doi: 10.1002/jbmr.2531. [DOI] [PubMed] [Google Scholar]
- 37.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendler DL, Zerbini C, Russo L, Greenspan S, Zikan V, Bagur A. Effects of 24 months treatment of teriparatide compared with risedronate on new fractures in postmenopausal women with severe osteoporosis: a randomized, double-dummy, clinical trial. Osteoporos Int. 2017;28(Suppl 1):69–70. [Google Scholar]
- 39.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. JAMA. 2016;316:722–33. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 40.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017 Sep 11; doi: 10.1056/NEJMoa1708322. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Rizzoli R, Cooper C, Reginster J-Y. Challenges for the development of bone forming agents in Europe. Calcif Tissue Int. 2014;94:469–473. doi: 10.1007/s00223-014-9844-9. [DOI] [PubMed] [Google Scholar]
- 42.Bone HG, Cosman F, Miller PD, Williams GC, Hattersley G, Hu M, Fitzpatrick LA, Papapoulos S. Sustained fracture risk reduction with sequential abaloparatide/alendronate: results of ACTIVEextend. J Bone Miner Res. 2017;32(Suppl 1):S25–26. abstract 1074. [Google Scholar]
- 43.Kanis JA, Cooper C, Abrahamsen B, Al-Daghri N, Brandi ML, Cannata-Andia J, et al. Identification and management of patients at increased risk of osteoporotic fracture. Osteoporos Int. 2017;28:2023–2034. doi: 10.1007/s00198-017-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]