Abstract
Background
The metabolic syndrome (MetS), as assessed using dichotomous criteria, is associated with increased risk of future chronic kidney disease (CKD), though this relationship is unclear among African Americans, who have lower risk for MetS but higher risk for CKD.
Methods
We performed logistic regression using a sex- and race-specific MetS-severity z-score to assess risk of incident CKD among 2,627 African-American participants of the Jackson Heart Study, assessed at baseline and 8 years later. Based on quartile of baseline MetS severity, we further assessed prevalence of being in the lowest quartile of baseline GFR, the lowest quartile of relative GFR at follow-up, microalbuminuria and incident CKD.
Results
Higher MetS-severity was associated with higher prevalence of GFR in the lowest quartile at baseline among males and females. Among African-American females but not males, higher baseline MetS-severity was associated with a higher prevalence of baseline elevations in microabuminuria (p<0.01), steep decline in GFR (p<0.001) and a higher incidence of CKD (p<0.0001). Women in increasing quartiles of baseline MetS-severity exhibited a linear trend toward higher odds of future CKD (p<0.05), with those in the 4th quartile of MetS-severity (compared to the 1st) having an odds ratio of 2.47 (95% confidence interval 1.13, 5.37); no such relationship was seen among men (p value for trend 0.49).
Conclusions
MetS-severity exhibited sex-based interactions regarding risk for future GFR deterioration and CKD, with increasing risk in women but not men. These data may have implications for triggering CKD screening among African-American women with higher degrees of MetS-severity.
Keywords: chronic kidney disease, metabolic syndrome, cardiovascular disease, glomerular filtration rate, microalbuminuria, risk
INTRODUCTION
Chronic kidney disease (CKD) represents a deterioration in kidney function, marked by low glomerular filtration rate (GFR), protein losses in the urine, and potential progression to end-stage renal disease (ESRD), with ultimate need for dialysis. CKD is influenced by underlying diabetes and is itself an independent risk factor for cardiovascular disease (CVD)[1]. African Americans are disproportionately affected by CKD and ESRD[2, 3], further raising the need to identify and characterize risk factors that could be used to initiate preventive measures. In addition, with loosening criteria for kidney donation, new tools are needed to assess usability of kidneys from patients of non-ideal health status[4].
One predictor of CKD risk is the metabolic syndrome (MetS), a cluster of cardiovascular risk factors including central obesity, high blood pressure (BP), high triglycerides, low HDL cholesterol and elevated fasting glucose[5, 6]. These abnormalities appear to be driven by common underlying processes such as altered adipocyte metabolism, systemic inflammation, oxidative stress and cellular dysfunction[7, 8]. MetS has traditionally been classified by the presence of abnormalities in at least 3 of the individual components, using criteria such as those of the Adult Treatment Panel-III (ATP-III)[5]. While MetS has primarily been assessed as a risk factor for type 2 diabetes and CVD, the presence of ATP-III MetS has been linked to risk for CKD, with odds ratios of 1.3–2.1 [9–12]. Importantly, these studies all identified this risk independent of baseline diabetes, highlighting the need to address MetS to help prevent future CKD[13].
Nevertheless, traditional MetS criteria have multiple limitations, including their binary classification and racial/ethnic discrepancies in which African Americans are under-diagnosed despite higher risk of diabetes, CVD and CKD[14–18]. We developed a sex- and race/ethnicity-specific MetS severity score[19, 20] that can be followed over time[21] and is linked to future risk of CVD[22, 23] and diabetes[24, 25] independent of the individual MetS components—overcoming many of the shortcomings of ATP-III MetS criteria. However, the relationship between the degree of MetS severity and future CKD is unclear.
The goals of the current study were to assess a prospective cohort of African Americans without diabetes at baseline for 1) the relationship between baseline MetS severity and subsequent CKD and 2) links between MetS severity, worsening GFR and microalbuminuria, both on a sex-specific basis. We hypothesized that higher MetS severity at baseline would be linked to subsequent development of CKD, lower GFR and higher urinary albumin-to-creatinine ratio. This study, the first to assess the severity of MetS and CKD risk, may have implications for clinical assessment of CKD risk in African American patients.
MATERIALS AND METHODS
The Jackson Heart Study Cohort
The Jackson Heart Study (JHS) is the largest longitudinal, single-site study of cardiovascular risk in African Americans. The cohort consists of 5,301 participants age 21–95 years [26]. We utilized data from JHS Visit 1 (2000–2004) and Visit 3 (2009–2013). During Visit 1, certified interviewers gathered information regarding income, education, and lifestyle factors, including tobacco smoking, dietary patterns and the amount of physical activity[26]. Participants had MetS components measured using standardized protocols[26–28]. Creatinine was determined via multipoint enzymatic spectrophotometric assay (Ortho-Clinical Diagnostics, Raritan, NJ)[28]. Urine albumin and creatinine was determined from spot urine samples on a subset of participants[29].
CKD assessments
CKD staging and severity was assessed by estimated GFR (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI)[30]:
where κ=0.7 if female and 0.9 if male; α=−0.329 if female and −0.411 if male; min=the minimum value of Serum Cr/κ or 1; max=the maximum value of Serum Cr/κ or 1; and age=years. CKD was defined as eGFR <60 mL/min/1.73 or on dialysis[31]. Overall kidney function assessments of interest between Visits 1 and 3 were CKD staging, binary classifications of CKD, continuous eGFR and relative decline in eGFR (calculated as Visit 3 GFR – Visit 1 GFR)/Visit 1 GFR * 100). Further, Visit 1 GFR and relative decline in GFR were both categorized into quartiles for each participant using the analytic sample in calculating reference cut-offs by quartile.
Urine albumin-to-creatinine ratio (ACR) was calculated. Microalbuminuria was defined as having ACR ≥30[29].
MetS severity assessment
Traditional MetS was defined using the ATP-III criteria for adults[5]; participants had to meet ≥3 of the following 5 criteria: concentration of triglycerides ≥150 mg/dL (1.69 mmol/L), HDL-C <40 mg/dL (1.04 mmol/L) for men and <50 mg/dL (1.3 mmol/L) for women, waist circumference (WC) ≥102 cm for males and 88 cm for females, glucose concentration ≥100 mg/dL (5.55 mmol/L), and systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg[5].
Continuous MetS severity z–scores at baseline were calculated for participants using sex- and race-based formulas. As described elsewhere [19, 20], these scores were derived using confirmatory factor analysis for the 5 traditional MetS components (WC, triglycerides, HDL-cholesterol, systolic BP, fasting glucose) to determine the weighted contribution of each component to a latent MetS “factor” on a sex- and race/ethnicity-specific basis. Confirmatory factor analysis was performed among adults 20–64 years from the National Health and Nutrition Examination Survey (NHANES) with categorization into six sub-groups based on sex and race/ethnicity (non-Hispanic-white, non-Hispanic-black and Hispanic). For each of these six population sub-groups, loading coefficients for the 5 MetS components were transformed into a single MetS factor and used to generate equations to calculate a standardized MetS severity score for each sub-group (http://mets.health-outcomes-policy.ufl.edu/calculator/). The resulting MetS-severity scores are z-scores (normally-distributed and ranging from theoretical negative to positive infinity with mean=0 and SD=1) of relative MetS severity on a sex- and race/ethnicity-specific basis. These scores have been validated in that they correlate strongly with other markers of MetS risk[32], including hsCRP[19], uric acid[19], the homeostasis model of insulin resistance[19], and adiponectin[33] and—more importantly—with long-term risk of CVD[22, 23] and diabetes[24, 25], including in the population in this analysis[24, 25].
Statistics
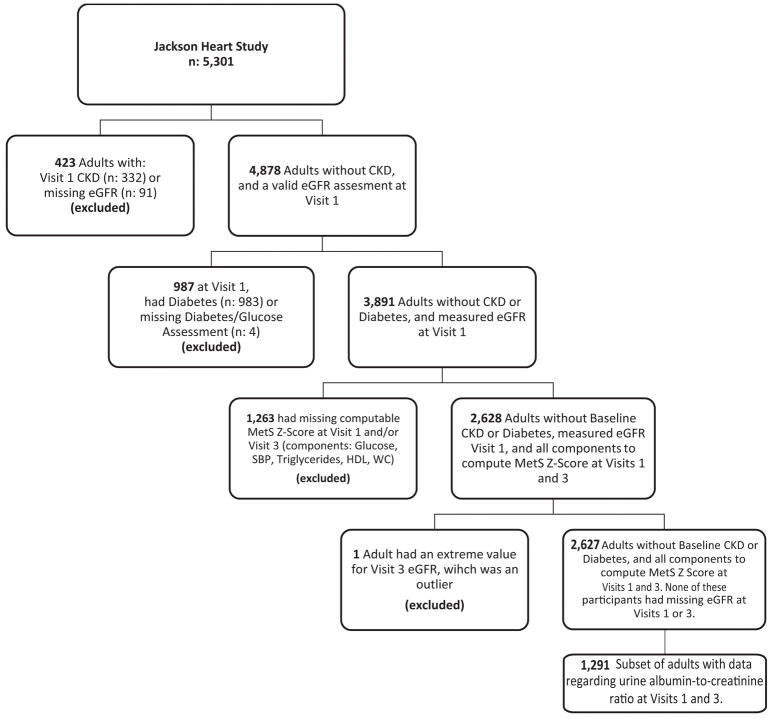
Participants with baseline CKD, diabetes, missing MetS components or eGFR were excluded from analysis (Figure 1). Because ACR was only available in a subset, analyses regarding the relationship between MetS severity and ACR were performed on a smaller analytic subset. Baseline descriptives for Visit 1 and Visit 3 were computed overall and by sex.
Figure 1.
Flow chart of participants included and excluded from analysis.
Percentiles of MetS severity, Visit-1 eGFR, and relative decline in eGFR (eGFR at Visit 3 relative to Visit 1) were computed for each participant. Given the MetS severity score is a z-score, percentiles correspond to the calculated z-score (i.e., these percentiles correspond to where participants would fit in the derivation cohort). Percentiles for the eGFR outcomes were calculated based on the analytic sample. Quartiles of Visit-1 MetS severity were examined for associations with Visit-1 eGFR <25th percentile, relative eGFR at Visit 3 <25th percentile, and CKD at Visit 3. The Cochran-Armitage test was used to test for trends across increasing MetS-severity quartiles for each outcome.
Logistic regression models were used to examine the odds of low Visit-1 eGFR (<25th percentile), rapid decline in eGFR between Visits 1 and 3 (<25th percentile of Visit 3 eGFR relative to Visit 1), and CKD at Visit 3. For each outcome, four primary models were used. The first utilized MetS-severity quartiles as a predictor to examine overall odds of the outcome; the second included a MetS x sex interaction to assess whether MetS-related associations differed between males and females. We then repeated these two modeling frameworks, using ATP-III MetS as a predictor. All models adjusted for age and sex; in the case of the Visit 3 outcomes, we also adjusted for Visit 1 eGFR. Lifestyle and sociodemographic variables found to be different between males and females were considered as confounders as well. Confounders were examined only for the model of odds of CKD at Visit 3 as a function of MetS severity (our primary model). We included all possible confounders after our initial comparisons in a fully-adjusted model, and removed in stepwise fashion those variables who were not significant predictors of the outcome in their own right and did not change any MetS odds ratio estimate by more than 15%. This was done in an iterative fashion on only those subjects with complete confounder data. The reference category for MetS-severity quartiles was <25th percentile. Rapid decline in eGFR was defined as a decline >16.8% of the Visit-1 GFR value. For models which used MetS-severity quartiles as a predictor, linear-trend p-values were assessed overall and by sex. Because of the small analytic subset with ACR measures and relatively few events of elevated ACR, regression analysis was not performed for this outcome. Statistical significance was classified as a p-value<0.05.
RESULTS
Participant characteristics
Table 1 displays participant characteristics for the 2627 individuals meeting inclusion criteria. Participants had mean age 52.8±12 years and mean BMI 31.2±7.1 kg/m2. ATP-III MetS was present in 30.3% of females and 24.4% of males (p=0.0008). MetS-severity z-score was −0.1±0.7 at baseline for both sexes. eGFR (ml/min/1.73 m2) at Visits 1 and 3, respectively, were 99.3±18.1 and 90.3±20.8 for females and 95.8±16.6 and 86.9±19.3 for males. In the subset with ACR measure (n=1291), the proportion with elevated ACR at Visits 1 and 3 was 5.5% and 6.1% for males and 6.9 and 7.4% for females. Over a mean 8.0 years of follow-up, ≥Stage 3 CKD developed in 7.4% of women and 8.6% of men.
Table 1.
Demographics
| n2 | Overall | Males | Females | p-value3 | ||
|---|---|---|---|---|---|---|
| Visit 1 | 2627 | 961 (36.6) | 1666 (63.4) | |||
| Age | 52.8 ± 11.9 | 52.2 ± 11.6 | 53.1 ± 12.1 | 0.07126 | ||
| BMI | 31.2 ± 7.1 | 29.4 ± 5.9 | 32.3 ± 7.5 | <0.00016 | ||
| Waist Circumference | 98.5 ± 15.7 | 99.4 ± 14.9 | 98.0 ± 16.2 | 0.03036 | ||
| Systolic Blood Pressure | 124.6 ± 16.9 | 126.5 ± 16.9 | 123.5 ± 16.9 | <0.00016 | ||
| Triglycerides | 97.3 ± 55.9 | 105.2 ± 64.6 | 92.7 ± 49.7 | <0.00016 | ||
| Fasting Glucose | 90.1 ± 8.9 | 91.1 ± 8.6 | 89.5 ± 9.0 | <0.00016 | ||
| HDL | 52.3 ± 14.4 | 46.6 ± 12.6 | 55.6 ± 14.4 | <0.00016 | ||
| ATP-III MetS | 741 (28.2) | 234 (24.4) | 507 (30.4) | 0.00083 | ||
| Smoker | 2602 | 306 (11.8) | 151 (15.9) | 155 (9.4) | <0.00013 | |
| Income Level | Poor | 2249 | 244 (10.9) | 55 (6.7) | 189 (13.3) | <0.00015 |
| Lower-Middle | 475 (21.1) | 139 (16.9) | 336 (23.6) | |||
| Upper-Middle | 705 (31.4) | 237 (28.8) | 468 (32.8) | |||
| Affluent | 825 (36.7) | 393 (47.7) | 432 (30.3) | |||
| Education | Less than HS | 2623 | 353 (13.5) | 140 (14.6) | 213 (12.8) | 0.89865 |
| HS | 448 (17.1) | 143 (14.9) | 305 (18.33) | |||
| Vocational | 793 (30.2) | 291 (30.3) | 502 (30.2) | |||
| College | 512 (19.5) | 215 (22.4) | 297 (17.9) | |||
| Graduate | 517 (19.7) | 170 (17.7) | 347 (20.9) | |||
| Physical Activity | Poor Health | 2625 | 1145 (43.6) | 404 (42.1) | 741 (44.5) | 0.00925 |
| Intermediate Health | 900 (34.3) | 303 (31.6) | 597 (35.9) | |||
| Ideal Health | 580 (22.1) | 253 (26.4) | 327 (19.6) | |||
| Nutrition | Poor Health | 1687 (64.2) | 653 (68.0) | 1034 (62.1) | 0.00225 | |
| Intermediate Health | 921 (35.1) | 303 (31.5) | 618 (37.1) | |||
| Ideal Health | 19 (0.7) | 5 (0.5) | 14 (0.8) | |||
| MetS Z-Score | −0.1 ± 0.7 | −0.1 ± 0.7 | −0.1 ± 0.7 | 0.89356 | ||
| Estimated GFR (EPI) | 98.04 ± 17.7 | 95.79 ± 16.6 | 99.33 ± 18.1 | <0.00016 | ||
|
| ||||||
| Visit 3 | 2627 | 961 (36.6) | 1666 (63.4) | |||
|
| ||||||
| MetS Z-Score | 0.1 ± 0.9 | 0.1 ± 0.8 | 0.2 ± 0.9 | 0.04876 | ||
| Estimated GFR (EPI) | 89.08 ± 20.4 | 86.92 ± 19.3 | 90.32 ± 20.8 | <0.00016 | ||
| Dialysis | 4 (0.2) | 2 (0.2) | 2 (0.1) | 0.62664 | ||
| Diabetes | 400 (15.2) | 148 (15.4) | 252 (15.1) | 0.85043 | ||
| Chronic Kidney Disease | 207 (7.9) | 83 (8.6) | 124 (7.4) | 0.27403 | ||
| CKD Staging | Stage 1 | 1259 (47.9) | 418 (43.5) | 841 (50.5) | 0.00085 | |
| Stage 2 | 1161 (44.2) | 460 (47.9) | 701 (42.1) | |||
| Stage 3 | 192 (7.3) | 78 (8.12) | 114 (6.8) | |||
| Stage 4 | 10 (0.4) | 3 (0.3) | 7 (0.4) | |||
| Stage 5 | 5 (0.2) | 2 (0.2) | 3 (0.2) | |||
|
| ||||||
| Subsample, Albumin:Creatinine | 1291 | 525 (40.7) | 766 (59.3) | |||
|
| ||||||
| Visit 1 ACR | 11.2 ± 22.8 | 9.7 ± 19.2 | 12.2 ± 24.9 | 0.03736 | ||
| Visit 1 Elevated ACR | 82 (6.4) | 29 (5.5) | 53 (6.9) | 0.31263 | ||
| Visit 3 ACR | 12.7 ± 24.9 | 11.4 ± 24.0 | 13.5 ± 25.6 | 0.12536 | ||
| Visit 3 Elevated ACR | 89 (6.9) | 32 (6.1) | 57 (7.4) | 0.34843 | ||
Presented: n(%) or mean ± standard deviation
If less than 2,627 total
Categorical: Chi-Square 2x2 tables
Categorical: Fisher’s Exact test, two-sided P
Categorical: Cochran-Mantel-Haenszel test (accounting for ordinality of the stage variable)
Continuous: t-test (some pooled some Satterthwaite, depending on equality of variances)
Relationship between MetS severity quartile and eGFR, eGFR decline and future CKD
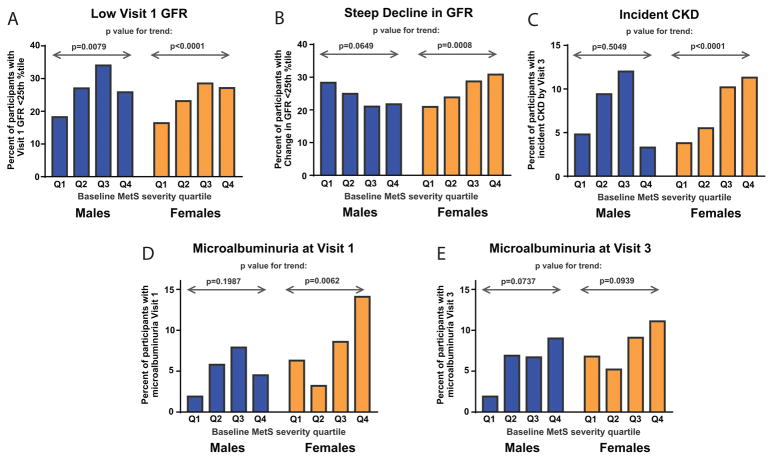
Table 2 and Figure 2 display proportions of four separate findings by baseline MetS severity quartile: 1) having eGFR <25th percent at baseline; 2) exhibiting a high relative decline of eGFR (<25th percentile of Visit 3 eGFR relative to Visit 1); 3) incident CKD by Visit 3; and 4) elevated ACR (in the subset with ACR measure). Overall results are displayed in Table 2 and results by sex in Figure 2. Increasing MetS-severity quartile was associated with the following: 1) a greater proportion of participants having a low baseline eGFR (p<0.01 in both sexes)(Figure 1A); 2) a higher proportion of having high relative decline in eGFR only in females, with a trend toward the opposite finding in males (p=0.065)(Figure 1B); 3) a higher proportion incident CKD in females (p<0.0001) but not males (p=0.505)(Figure 2C); and 4) a greater proportion of elevated ACR overall at Visits 1 and 3, which on a sex-specific basis was only present in females at Visit 1.
Table 2.
Examination of Visit 1 MetS Severity with a) Visit 1 eGFR, b) Visit 3 CKD, and c) relative GFR decline
| n | Visit 1 eGFR < 25th percentile (< 85.1) | CKD at Visit 3 | Relative eGFR decline* (%) < 25th percentile (< 16.8%) |
|
|---|---|---|---|---|
|
|
|
|
|
|
| Overall | 2627 | |||
| Visit 1 MetS Severity Percentile** | ||||
| < 25th | 560 | 95 (17.0%) | 23 (4.1%) | 131 (23.4%) |
| 25–50th | 855 | 210 (24.6%) | 60 (7.0%) | 207 (24.2%) |
| 50th–75th | 826 | 253 (30.6%) | 90 (10.9%) | 212 (25.7%) |
| > 75th | 386 | 103 (26.7%) | 34 (8.8%) | 108 (28.0%) |
| p-value*** | < 0.0001 | < 0.0001 | 0.0889 |
Relative decline (percent) = (Visit 3 GFR − Visit 1 GFR)/Visit 1 GFR * 100
MetS percentile is based on the z-score value (i.e., from the derivation cohort)
Cochran-Armitage trend test p-value
Figure 2. Relationship between baseline MetS severity quartile and prevalence of renal outcomes by sex.
Data shown display prevalence of: A. having baseline eGFR in lowest 25 perecentile, B. high relative decline in eGFR (Visit 3 eGFR relative to Visit 1 eGFR in the lowest 25 percentile), C. incident CKD by Visit 3, D. microalbuminuria at Visit 1, and E. microalbuminuria at Visit 3. P value for trend in proportion by MetS severity quartile is shown for males and females.
Logistic regression of MetS severity and low baseline GFR
Table 3 provides odds of low eGFR (<85.1, 25th percentile) at Visit 1 from models that looked at overall odds (first set of columns) as well as sex-specific odds (via a MetS x sex interaction and shown in columns by sex), done separately for both MetS-severity quartile (Model 1) and ATP-III MetS. Our confounding analysis led to final adjustment by Visit-1 age, physical activity, nutrition, and current smoking status. Income was not found to be a confounder (i.e., its inclusion in the models did not change the odds ratio estimates) and was not associated with low eGFR by itself, so it was not included in these final models. In assessing MetS-severity (with <25th percentile as the referent), the overall model revealed a tendency for higher odds of low baseline eGFR for ascending quartiles, though this was significant only for the 3rd MetS-severity quartile (OR=1.53 [1.14, 2.05]). The model that included a MetS x sex interaction clarified that odds of low baseline GFR were higher for males in the 3rd MetS-severity quartiles (OR 1.97 [1.23, 3.17]). Among females in this model, there was no significant relationship between MetS severity quartile and low baseline eGFR.
Table 3.
Logistic Regression Models of Low Visit 1 GFR (< 85.1, 25th percentile): MetS Severity vs. ATP-III MetS*
| Overall Odds Ratio of Low Visit 1 GFR | Sex-Specific Odds Ratios of Low Visit 1 GFR | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds Ratio (95% CI) | p-value | Male Odds Ratio (95% CI) | p-value | Female Odds Ratio (95% CI) | p-value | |
| Model with Visit 1 MetS Severity (Quartiles)** | ||||||
| 25–50th | 1.32 (0.99, 1.78) | 0.0614 | 1.56 (0.96, 2.52) | 0.0732 | 1.21 (0.83, 1.75) | 0.3217 |
| 50–75th | 1.53 (1.14, 2.05) | 0.0041 | 1.97 (1.23, 3.17) | 0.0052 | 1.31 (0.90, 1.89) | 0.1569 |
| > 75th | 1.20 (0.85, 1.70) | 0.3030 | 1.52 (0.83, 2.81) | 0.1790 | 1.05 (0.69, 1.61) | 0.8162 |
| Linear trend p-value | 0.2046 | 0.1386 | 0.7320 | |||
| Interaction p-value = 0.5924 | ||||||
| Model with ATP-III MetS | Odds Ratio (95% CI) | p-value | ||||
|
|
||||||
| Visit 1 ATP-III MetS | 1.26 (1.02, 1.55) | 0.0313 | 1.21 (0.86, 1.72) | 0.2922 | 1.28 (0.99, 1.66) | 0.0579 |
| Interaction p-value = 0.7874 | ||||||
Four models were fit. Two models across all subjects were fit, one with Visit 1 MetS severity and one with ATP-III MetS (top half and bottom half of the first set of columns). These models then were re-fit to include an interaction with sex to allow for sex-specific examination of the association between MetS and Low Visit 1 GFR (second set of columns, by sex). For all four models, the following covariates were included: Visit 1 age, sex, physical activity, nutrition, and current smoking status. Differences in income were observed between males and females, but income was not found to be a confounder (i.e., did not change above estimates) and was thus removed from the models given large amounts of missing data on this variable.
Reference category: < 25th percentile.
The model with ATP-III MetS (vs. not) had a significantly higher odds of low baseline eGFR (OR=1.26 [1.02, 1.55]) that was not statistically-significant when considered on a sex-specific basis.
Logistic regression of MetS severity and future CKD
The top half of Table 4 provides model results of odds of CKD at Visit 3, with the same adjustments as described above, with the added adjustment of Visit 1 eGFR. For the overall sample, participants in the 3rd MetS-severity quartile (vs. the 1st) had a higher odds of future CKD (OR=1.88 [1.09, 3.25]). On a sex-specific basis, there was a higher odds of future CKD for women in the 4th quartile (OR=2.47 [1.13, 5.37]) and a significant trend of increasing OR’s for increasing MetS-severity quartiles (p=0.01). No associations or trends were observed among males.
Table 4.
| Overall Odds Ratio of Visit 3 Kidney Disease | Sex-Specific Odds Ratios of Visit 3 Kidney Disease | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds Ratio (95% CI) | p-value | Male Odds Ratio (95% CI) | p-value | Female Odds Ratio (95% CI) | p-value | |
| Outcome: Incident CKD at Visit 3 | ||||||
| Model with Visit 1 MetS Severity (Quartiles) | ||||||
| 25–50th | 1.58 (0.90, 2.79) | 0.1124 | 2.01 (0.85, 4.77) | 0.1134 | 1.31 (0.61, 2.78) | 0.4903 |
| 50–75th | 1.88 (1.09, 3.25) | 0.0231 | 1.98 (0.85, 4.61) | 0.1126 | 1.81 (0.89, 3.72) | 0.1036 |
| > 75th | 1.07 (0.99, 3.50) | 0.0556 | 0.63 (0.17, 2.34) | 0.4876 | 2.47 (1.13, 5.37) | 0.0229 |
| Linear trend p-value | 0.1227 | 0.4933 | 0.0128 | |||
| Interaction p-value = 0.0645 | ||||||
| Model with ATP-III MetS | Odds Ratio (95% CI) | p-value | ||||
|
|
||||||
| Visit 1 ATP-III MetS | 1.30 (0.93, 1.84) | 0.1289 | 1.07 (0.60, 1.92) | 0.8111 | 1.45 (0.95, 2.22) | 0.0878 |
| Interaction p-value = 0.4153 | ||||||
|
| ||||||
| Outcome: Rapid Decline of eGFR (Visit 1 to Visit 3 > −16.8% of Visit 1) | ||||||
| Model with Visit 1 MetS Severity (Quartiles) | ||||||
| 25–50th | 0.97 (0.75, 1.26) | 0.8072 | 0.87 (0.57, 1.32) | 0.5047 | 1.03 (0.74, 1.43) | 0.8795 |
| 50–75th | 0.96 (0.74, 1.24) | 0.7354 | 0.64 (0.42, 0.99) | 0.0444 | 1.20 (0.86, 1.67) | 0.2768 |
| > 75th | 1.03 (0.76, 1.41) | 0.8310 | 0.75 (0.43, 1.30) | 0.2999 | 1.22 (0.84, 1.79) | 0.2947 |
| Linear trend p-value | 0.8568 | 0.6939 | 0.2023 | |||
| Interaction p-value = 0.0862 | ||||||
| Model with ATP-III MetS | Odds Ratio (95% CI) | p-value | ||||
|
|
||||||
| Visit 1 ATP-III MetS | 1.06 (0.86, 1.29) | 0.5919 | 0.97 (0.68, 1.39) | 0.8846 | 1.10 (0.86, 1.40) | 0.4521 |
| Interaction p-value = 0.5853 | ||||||
Two outcomes were utilized: CKD at Visit 3, and Rapid Decline of eGFR (Visit 1 to Visit 3 > −16.8 percent of Visit 1).
For each of the two outcomes, four models were fit. Two models across all subjects were fit, one with Visit 1 MetS severity and one with ATP-III MetS (first set of columns, divided between incident CKD as an outcome in the upper half of table and rapid eGFR decline in the bottom half of table). These models then were re-fit to include an interaction with sex to allow for sex-specific examination of the association between MetS and Low Visit 1 GFR. For all eight models, the following covariates were included: Visit 1 age, Visit 1 eGFR, sex, physical activity, nutrition, and current smoking status. Differences in income were observed between males and females, but income was not found to be a confounder (i.e., did not change above estimates) and was thus removed from the models given large amounts of missing data on this variable.
Reference category: < 25th percentile.
Participants with ATP-III MetS at baseline did not have greater odds of later CKD, either overall or on a sex-specific basis.
Logistic regression of MetS severity and greater decline in GFR
The bottom half of Table 4 provides odds of having a high relative decline in eGFR between Visits 1 and 3 (>16.8%) for MetS-severity quartile and ATP-III MetS, both overall and by sex (with the same adjustments as the models of Visit 3 CKD odds). There was no significant relationship between MetS-severity quartile and odds of high relative decline of eGFR for the overall or among females (p-value for trend =0.20). Among males, there was a surprising protective effect of being in the 3rd MetS-severity quartile relative to the 1st quartile (OR=0.64 [0.42, 0.94]).
Participants with ATP-III MetS (vs. not) did not have a greater odds of exhibiting high relative decline in eGFR, overall or on a sex-specific basis.
DISCUSSION
Whereas prior studies evaluated for CKD risk associated with dichotomous MetS criteria[9–12], we used a sex- and race/ethnicity-specific MetS-severity z-score to evaluate relationships between the degree of MetS severity and risk for progression to CKD in an African-American cohort without baseline diabetes. Baseline MetS severity exhibited an expected inverse relationship with baseline eGFR in both sexes, as seen previously using ATP-III criteria[34]. Intriguingly, however, we found sex differences in longitudinal relationships with MetS severity, with females (but not males) exhibiting correlations between baseline MetS severity and both subsequent worsening eGFR and progression to CKD. The extent of these relationships among females suggests potential clinical benefit of following MetS severity over time among African-American women. Just as higher baseline MetS severity and greater rise over time has been associated with risk for future CVD[22, 23] and diabetes[24, 25], this higher risk for CKD could prompt evaluation for worsening kidney function—potentially identifying disease at an earlier time point. These findings are important, particularly because this could then prompt earlier treatment of CKD among African-American women, who are disproportionately affected with CKD and progression to ESRD. Additionally, a score such as this may have implications regarding organ donation acceptability from individuals with otherwise borderline status based on BP, BMI or eGFR—with higher MetS severity suggesting increased underlying damage raising potential risk for ultimate deterioration of function, even in a recipient without MetS[4].
Among males, we were surprised to find that men in the highest quartile of baseline MetS severity appeared if anything protected against later CKD (Figure 2C). There was a similar lack of association of ATP-III MetS with future CKD in men, which is notable, as African-American men have not been highly represented in prior MetS-CKD studies. We also noted a trend (p=0.065) toward an unusual paradoxical association of higher baseline MetS severity being associated with less deterioration in eGFR among men (Figure 2B). These findings were surprising and may be hypothesis generating. One hypothesis is that this reflects hyperfiltration, in which the kidney compensates for increased metabolic demands, as noted in obesity[35], insulin resistance[36], MetS[37], and subclinical CVD[38]. This could increase GFR, with initial decrease in CKD. Ultimately, however, the etiology of these sex differences remains unknown, as men are overall more likely than women to progress to CKD[39]. Another hypothesis is that African-American men experience a greater proportion of non-MetS causes of CKD. Nevertheless, the sex-specific differences in our analyses persisted following adjustment for CKD risk factors such as smoking [40], low physical activity and poor nutrition[41] which were more common among men in this cohort. Another hypothesis is that these sexually dimorphic findings are related more so to obesity, as women in this cohort had a higher prevalence of obesity, an independent risk factor for development of CKD[42].
The NIH has encouraged sex-specific analyses because of growing appreciation of diverging physiological/pathophysiological effects of many processes by sex, including for renal pathology. While men are overall more likely to progress to CKD[39], women have been noted to be at both higher[43] and lower[44] risk of acute kidney injury (AKI) following cardiac surgery, depending on the AKI criteria used. Additionally, a Dutch study of white individuals (mean age 49 years) found that BMI and plasma glucose had a greater relationship with the degree of urinary albumin excretion among men than among women[45]. Finally, with respect to MetS and CKD, a prior cross-sectional analysis of a Chinese cohort found that ATP-III MetS was more strongly associated with lower eGFR in males compared to females[34]. While causative etiologies remain elusive, these studies support the potential for sex differences in the progression of renal disease. Nevertheless, multiple longitudinal cohort studies in white and Asian cohorts have not reported sex differences in ATP-III MetS as a predictor of future CKD[9–12] including a meta-analysis of such studies[6]. This could mean that this relationship is not present in other racial/ethnic groups or that ATP-III MetS, in addition to its known racial/ethnic discrepancies[15–17], is not as effective at detecting sex-based differences.
We did not observe MetS-related findings using ATP-III MetS in this cohort, as noted elsewhere[46]. A prior cross-sectional study from JHS Visit 1 determined that ATP-III MetS carried an OR of 2.2 for current CKD[47]. However, that analysis did not exclude diabetes, raising the potential for confounding between the effects of diabetes and the abnormalities related to MetS—either from the pathological processes underlying MetS[7, 8] or the individual MetS components. Indeed, the prior study found certain combinations of MetS abnormalities conferred stronger risk than others, including multiple combinations involving glucose elevations[47]. We excluded participants with baseline diabetes to assess for CKD risk in the absence of extreme baseline glucose elevations. We noted similar prevalence of incident diabetes by sex. That baseline MetS severity remained linked with CKD among women suggests risk associated with MetS severity that was not captured by dichotomous ATP-III MetS criteria.
MetS appears from prior studies to contribute to renal damage through a variety of mechanisms, including secretion of angiotensinogen by visceral adipocytes[48], lipotoxicity from high levels of triglycerides[49], sodium retention from insulin resistance[13] and glomerular damage from oxidative stress[50], as well as ongoing effects of hypertension. However, it is unclear how these processes may differ between sexes, requiring further investigation.
This study had several limitations. We evaluated data from a large cohort in the Jackson area of Mississippi, and it is unclear whether these results can be generalized to African-American individuals from other areas. Assessment of GFR using creatinine has limitations, leading to use of additional factors such as cystatin C, which was unavailable in this cohort. We had ACR measures in only a subset of participants, limiting more detailed analysis of findings between MetS and microalbuminuria. We lacked analysis regarding relationships between CKD and MetS-related non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of MetS, which in prior studies is independently linked to CKD[51] but which is less tightly linked to MetS among African Americans[52]. However, this study had multiple strengths, including longitudinal follow of a large cohort of African Americans, who have been missing from prior analyses of the relationships between MetS and CKD.
In conclusion, we found in this cohort of African-American individuals that while MetS severity exhibited the expected inverse association with eGFR at baseline in both sexes, the association between MetS severity and deterioration of eGFR to CKD was seen only in women. In addition, worsening of MetS severity over time was further tied to decline in GFR, raising the potential for following MetS severity over time in surveillance for worsening kidney status—and potentially as a motivator toward preventative lifestyle changes.
Acknowledgments
Funding: This work was supported by NIH grants 1R01HL120960 (MJG and MDD). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Footnotes
Conflict of Interest Statement: None of the investigators has any conflicts of interest related to this investigation to disclose. The results of these analyses, in whole or in part, have not been published previously.
Authors’ Contributions: Research idea and study design: MDD, MDO, MJG; statistical analysis: SLF, MJG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 3.Evans K, Coresh J, Bash LD, Gary-Webb T, Köttgen A, Carson K, Boulware LE. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant. 2011;26:899–908. doi: 10.1093/ndt/gfq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong G, Craig JC, Chapman JR. Setting the limit for living kidney donation-how big is too big? Kidney Int. 2017;91:534–536. doi: 10.1016/j.kint.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2237–2238. doi: 10.1056/NEJMc1412427. [DOI] [PubMed] [Google Scholar]
- 8.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 10.Ryu S, Chang Y, Woo HY, Lee KB, Kim SG, Kim DI, Kim WS, Suh BS, Jeong C, Yoon K. Time-dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am J Kidney Dis. 2009;53:59–69. doi: 10.1053/j.ajkd.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Lucove J, Vupputuri S, Heiss G, North K, Russell M. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kidney Dis. 2008;51:21–28. doi: 10.1053/j.ajkd.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya T, Kiyohara Y, Kubo M, Yonemoto K, Tanizaki Y, Doi Y, Hirakata H, Iida M. Metabolic syndrome and CKD in a general Japanese population: the Hisayama Study. Am J Kidney Dis. 2006;48:383–391. doi: 10.1053/j.ajkd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal V, Shah A, Rice C, Franklin BA, McCullough PA. Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol. 2009;5:520–528. doi: 10.1038/nrneph.2009.114. [DOI] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutrition Metabolism and Cardiovascular Diseases. 2012;22:141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999–2006. Metabolism. 2012;61:554–561. doi: 10.1016/j.metabol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBoer MD. Underdiagnosis of Metabolic Syndrome in Non-Hispanic Black Adolescents: A Call for Ethnic-Specific Criteria. Curr Cardiovasc Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurka MJ, Lilly CL, Norman OM, DeBoer MD. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovascular Diabetology. 2012:11. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vishnu A, Gurka MJ, DeBoer MD. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: The Atherosclerosis Risk in Communities Study. Atherosclerosis. 2015;243:278–285. doi: 10.1016/j.atherosclerosis.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Amer Coll Card. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeBoer MD, Gurka MJ, Hill Golden S, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent Associations between Metabolic Syndrome Severity & Future Coronary Heart Disease by Sex and Race. J Am Coll Card. 2017;69:1204–1205. doi: 10.1016/j.jacc.2016.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Cardel M, Pearson TA, DeBoer MD. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia. 2017;60:1261–1270. doi: 10.1007/s00125-017-4267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor HA, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 27.Taylor H, Liu J, Wilson G, Golden SH, Crook E, Brunson CD, Steffes M, Johnson WD, Sung JH. Distinct component profiles and high risk among African Americans with metabolic syndrome: the Jackson Heart Study. Diabetes Care. 2008;31:1248–1253. doi: 10.2337/dc07-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, Fülöp T, Bansal N, Robinson-Cohen C, Griswold M, Powe NR, Himmelfarb J, Correa A. Risk Factors for Rapid Kidney Function Decline Among African Americans: The Jackson Heart Study (JHS) Am J Kidney Dis. 2016;68:229–239. doi: 10.1053/j.ajkd.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J Collaboration) C-ECKDE. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens PE, Levin A Members KDIGOCKDGDWG. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 32.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the national health and nutrition examination survey 1999–2006. Metab Syndr Relat Disord. 2010;8:343–353. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016 doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen J, Guo CX, Lu MG, Lu Y, Huang Y, Liu X, Li Y, Huang ZJ, Zhang YP, Yuan H. Gender-specific association between metabolic syndrome and decreased glomerular filtration rate in elderly population. Int Urol Nephrol. 2016;48:389–397. doi: 10.1007/s11255-015-1172-0. [DOI] [PubMed] [Google Scholar]
- 35.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 36.Lee AM, Charlton JR, Carmody JB, Gurka MJ, DeBoer MD. Metabolic risk factors in nondiabetic adolescents with glomerular hyperfiltration. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, Sattar N, Zukowska-Szczechowska E, Dominiczak AF. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007;71:816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 38.Eriksen BO, Løchen ML, Arntzen KA, Bertelsen G, Eilertsen BA, von Hanno T, Herder M, Jenssen TG, Mathisen UD, Melsom T, Njølstad I, Solbu MD, Toft I, Mathiesen EB. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014;86:146–153. doi: 10.1038/ki.2013.470. [DOI] [PubMed] [Google Scholar]
- 39.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 40.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 41.MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55:69–76. doi: 10.1053/j.ajkd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg. 2008;107:239–249. doi: 10.1213/ane.0b013e318178ca42. [DOI] [PubMed] [Google Scholar]
- 44.Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the Risk of AKI Following Cardio-thoracic Surgery: A Meta-Analysis. Clin J Am Soc Nephrol. 2016;11:2113–2122. doi: 10.2215/CJN.03340316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verhave JC, Hillege HL, Burgerhof JG, Navis G, de Zeeuw D, de Jong PE, Group PS. Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol. 2003;14:1330–1335. doi: 10.1097/01.asn.0000060573.77611.73. [DOI] [PubMed] [Google Scholar]
- 46.Navaneethan SD, Schold JD, Kirwan JP, Arrigain S, Jolly SE, Poggio ED, Beddhu S, Nally JV., Jr Metabolic Syndrome, ESRD, and Death in CKD. Clinical Journal of the American Society of Nephrology. 2013;8:945–952. doi: 10.2215/CJN.09870912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendy VL, Azevedo MJ, Sarpong DF, Rosas SE, Ekundayo OT, Sung JH, Bhuiyan AR, Jenkins BC, Addison C. The association between individual and combined components of metabolic syndrome and chronic kidney disease among African Americans: the Jackson Heart Study. PLoS One. 2014;9:e101610. doi: 10.1371/journal.pone.0101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 50.Sarafidis PA, Whaley-Connell A, Sowers JR, Bakris GL. Cardiometabolic syndrome and chronic kidney disease: what is the link? J Cardiometab Syndr. 2006;1:58–65. doi: 10.1111/j.0197-3118.2006.05470.x. [DOI] [PubMed] [Google Scholar]
- 51.Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, Zenari L, Bonora E. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53:1341–1348. doi: 10.1007/s00125-010-1720-1. [DOI] [PubMed] [Google Scholar]
- 52.Deboer MD, Wiener RC, Barnes BH, Gurka MJ. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics. 2013;132:e718–726. doi: 10.1542/peds.2012-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]