Figure. 3. Comparison of Bucky ball/XVelo/Oskar functional domains.
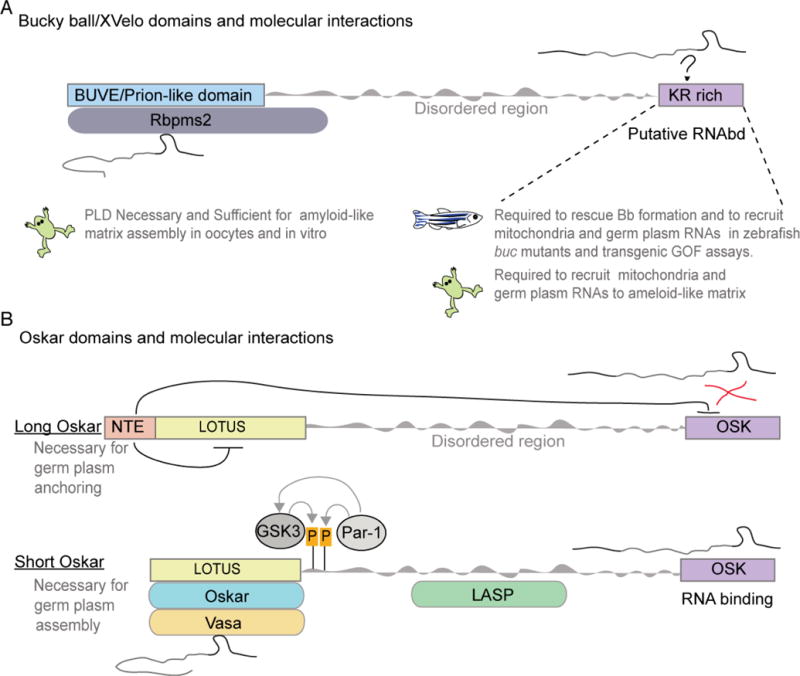
A) Schematic depicting the protein domains of Xenopus Velo and zebrafish Bucky ball. The amino terminus harbors a prion-like domain (PLD), also known as the BUVE (Bucky ball Velo domain) that is essential for assembly of an amyloid-like matrix. The Carboxy terminus is K/R rich and has properties suggestive of RNA binding function. The C-terminus is required for Balbiani body assembly in zebrafish, including recruiting RNAs and mitochondria, and for entrapment of mitochndria and RNAs in XVelo assembled amyloid-like matrix in Xenopus oocytes and reconstitution assays. The PLD and KR rich region are separated by an intrinsically disorded region. B) Schematic depicting long and short forms of Drosophila Oskar proteins. The Amino termimal extension (NTE) of the long isoform is essential for nucleating actin filaments and anchoring of germ plasm components to the posterior cortex in oocytes. The LOTUS domain mediates Oskar dimerization and interactions with protein binding partners, like Vasa. The actin binding protein Lasp binds to the disordered region. The OSK domain is composed of basic and hydrophilic amino acids and is thought to mediate interactions with the RNA effectors of Oskar. Short Oskar activity and stabiity are regulated by posttranslational modifications, including phosphorylation and ubiquitination (not shown). Although long Oskar has the LOTUS and OSK domains it does not bind to Vasa nor does it bind to RNAs. The NTE is thought to inhibit these activities.
