Figure. 4. Mitochondria asymmetry, Balbiani body formation, and germ plasm assembly as potential oocyte bottlenecks.
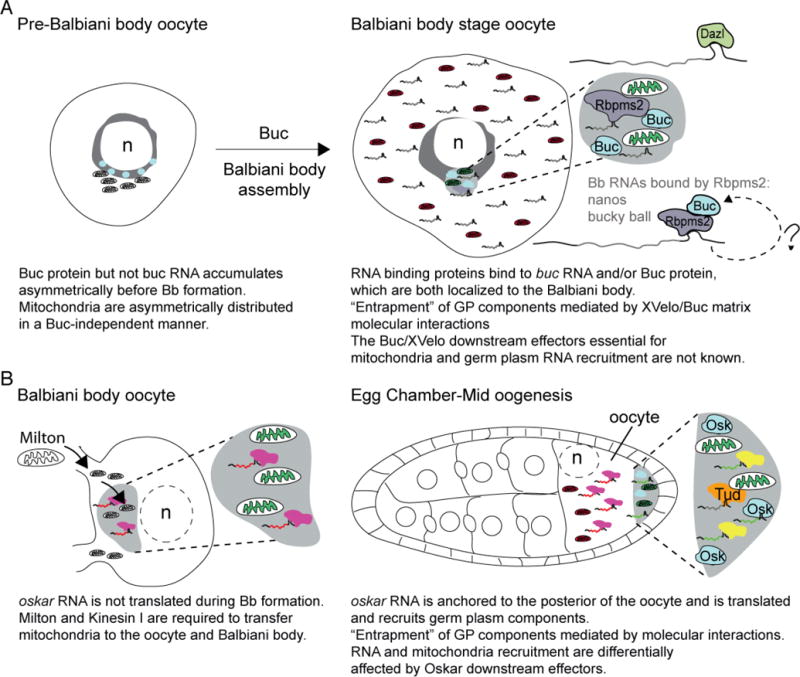
A) Schematic depicts an early zebrafish oocyte prior to Balbiani body formation. Buc protein is asymmetric next to the nucleus, and mitochondria are asymmetrically distributed in oocytes in a Buc-independent manner at this stage. Buc is required for Balbiani body assembly and recruitment of the most active mitochondria (green) there. RNAs and proteins, including RNA binding proteins (like Dazl and Rbpms2) are also recruited to the Balbiani body at this time. Although the mechanism of recruitment is not understood, a mechanism whereby Buc forms a self-assembling network that can recruit germ plasm (GP) components directly, and through conserved interactions with RNA binding proteins has been proposed for Balbiani body assembly in zebrafish and Xenopus oocytes. Analysis of zebrafish maternal-effect mutants and overexpression assays indicates that Buc/XVelo coordinate recruitment, possibly by entrapment, of mitochondria and RNAs to the nonmembrane bound Balbiani body. B) In Drosophila, one of 16 cyst cells is specified as the oocyte. Mitochondria are transferred to the oocyte in a Kinesin and Milton dependent manner to form the Balbiani body. At this stage, oskar RNA is present, but is translationally repressed; therefore, if recruitment of mitochondria at this stage requires oskar this would be an RNA function rather than a coding function of oskar. In mid-oogenesis, oskar RNA is anchored at the posterior pole and is translated there. Once translated, short Oskar mediates germ plasm assembly components, including mitochondria via self-assembly and interactions with its RNA and protein effectors, including RNA binding proteins that lead to entrapment of components within the germ plasm. Tudor (Tud) binds to mitochondria and RNA, but is not required to localize mitochondria to the posterior pole. Therefore, in Drosophila recruitment of mitochondria and germ plasm RNAs to nonmembrane bound compartments can be uncoupled by Oskar downstream effectors. Balbiani body formation is a conserved feature of oocyte development and analysis of mitochondria indicates bottlenecks within these stages; however, it remains to be determined if selection at either of these stages influences mitochondrial content of oocytes or the embryonic germline.
