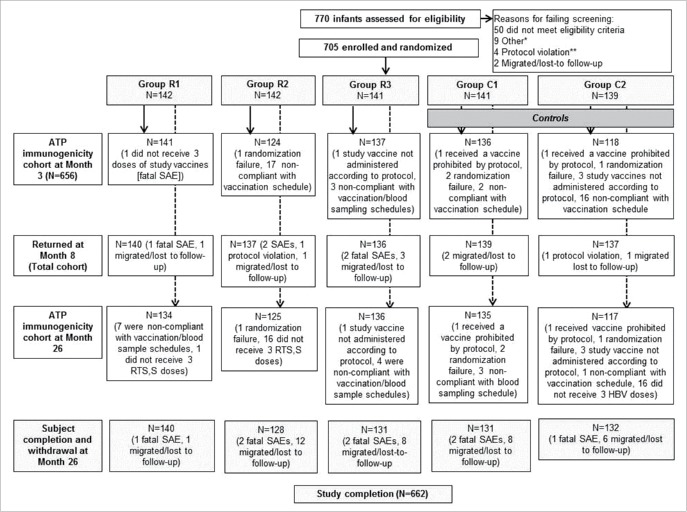
Figure 1.
Study flow per co-administration vaccination regimen from week 0 until month 26.Group R1 received RTS,S/AS01 + (DTaP/Hib + tOPV + PHiD-CV), and HRV 2 weeks later, Group R2 received RTS,S/AS01 + (DTaP/Hib + tOPV + HRV), and PHiD-CV 2 weeks later, Group R3 received RTS,S/AS01 + (DTaP/Hib + tOPV), and (PHiD-CV + HRV) 2 weeks later, Group C1 received HBV + (DTaP/Hib + tOPV + PHiD-CV), and HRV 2 weeks later, Group C2 received HBV + (DTaP/Hib + tOPV + HRV), and PHiD-CV 2 weeks later, * Other: five consent withdrawal, two recruitment target reached in SBIR, one down syndrome, and one end of inclusion (recruitment was completed), ** Protocol violation: three screening expired and one child received recommended vaccines before enrolment, DTaP/Hib = diphtheria-tetanus-acellular-pertussis-Haemophilus influenzae type-b-conjugate vaccine, tOPV = trivalent oral poliovirus vaccine, PHiD-CV = 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine, HRV = human rotavirus vaccine.