Figure 6.
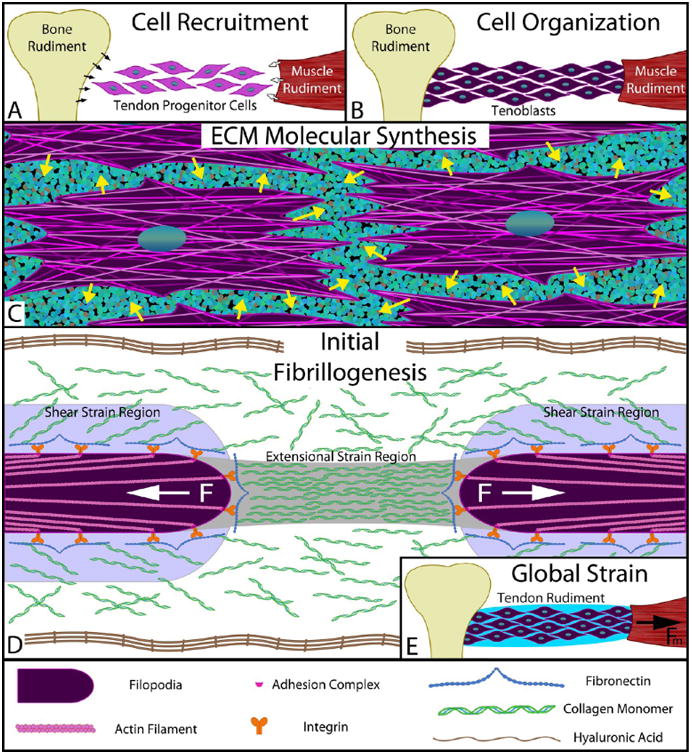
Simplified model of early stage of tendon morphogenesis in vivo. (A) Recruitment: Tendon progenitor cells express signaling molecules, such as TGFβ,65 which recruit additional cells from the cartilaginous bone rudiment (black arrows) and the muscle rudiment (white arrows). (B) Migration/organization: Recruited cells migrate into the prospective tendon space between the bone and muscle rudiments, organize to the long axis of the tendon, and take on a “tenoblastic” identity. (C) Synthesis: “Tenoblast” cells discharge structural molecules into the extracellular space (e.g., collagen monomers, fibronectin, elastin, and proteoglycans) as well as hyaluronic acid to molecularly crowd the environment surrounding the cells. (D) Discrete segment fibrillogenesis: Filopodia on the fibroblasts express collagen-binding complexes (e.g., α5β1–fibronectin–collagen) and exert a contractile force via nonmuscle myosin II. The flow field around the retracting filopodia creates both extensional and shear strains within the surrounding chemical milieu, which align the crowded collagen molecules and trigger FIC to produce fibril segments. Our data indicate that the retraction can produce extensional strains sufficient to cause FIC for ∼10 μm (see Figure S8a). Each filopodia retracts until a stalling force resistance is met or until complete contraction of the actin filament occurs, at which point an invagination in the cell membrane may result. During this phase of morphogenesis, local cell contraction should manifest in the form of short, disconnected fibrils as seen in early tendon morphogenesis.76 (E) Drive to continuity: Tissue strains, imposed through the contraction of the muscle rudiment, preferentially induce amplified extensional strain rates (see eq 1 and Supporting Information on extensional strain rate magnification) in the gaps between cells and between fibril segments. This triggers the formation of additional collagen fibrils precisely where they are required for connectivity. As fibril segments fuse, gaps shrink and the extensional strains are further amplified. FIC continues to occur under the continually increasing extensional strain rates until continuity of load-bearing structure is converged upon. The model proposed is consistent with most observations of tendon morphogenesis, particularly with regard to fibrillogenesis.
