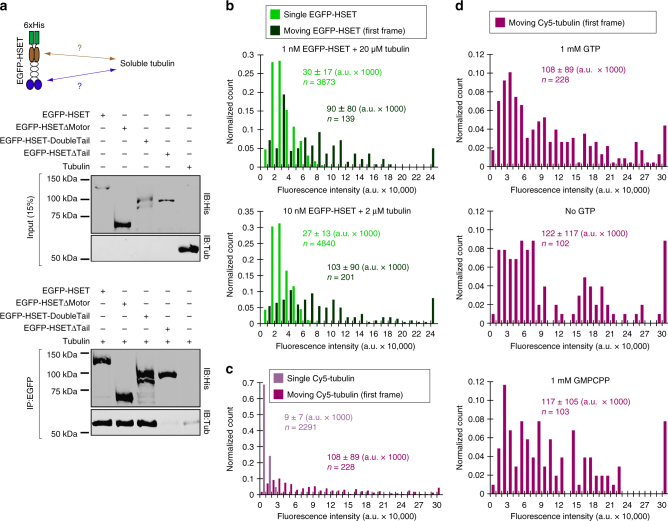
Fig. 3.
Soluble (non-MT) tubulin binding to N-terminal HSET tail domain induces HSET-tubulin clustering to activate long-range unidirectional motion. a Co-immunopreciptation of purified HSET with soluble tubulin. The indicated EGFP-HSET truncation (50 nM concentration) was incubated with soluble tubulin (250 nM concentration) and the common EGFP-tag was used for immunoprecipitation. Inputs were loaded at 15% of total protein. A representative blot from N = 2 independent experiments is shown. b Fluorescence intensity analysis of EGFP-HSET diluted to single-molecule levels and adhered to a glass cover slip (light green) compared to the first frame of moving EGFP-HSET after the addition of tubulin (dark green, concentrations indicated). The fluorescence intensity of individual EGFP-HSET particles was determined by Gaussian fit, and intensity distributions were plotted as histograms for the population, where normalized count is the observed value in the bin divided by the total number of particles. Data are reported as the arithmetic mean ± SD for the indicated n particles from 2 independent experiments, where N ≥ 4 movies for each condition. c, d Fluorescence intensity analysis of Cy5-tubulin diluted to single-molecule levels and adhered to a glass cover slip (light purple) compared to the first frame of moving Cy5-tubulin (10 nM) in the presence of 10 nM unlabeled HSET (dark purple). The fluorescence intensity of individual Cy5-tubulin particles was determined by Gaussian fit, and intensity distributions were plotted as histograms for the population. Data are reported as the arithmetic mean ± SD for the indicated n particles from 2 independent experiments where N = 6 movies for moving Cy5-tubulin. For d, fluorescence intensities were determined in the presence of the indicated guanine nucleotide, where N ≥ 2 movies for each condition. All experiments were performed in BRB80 + 50 mM KCl with 0.5 mg/mL casein