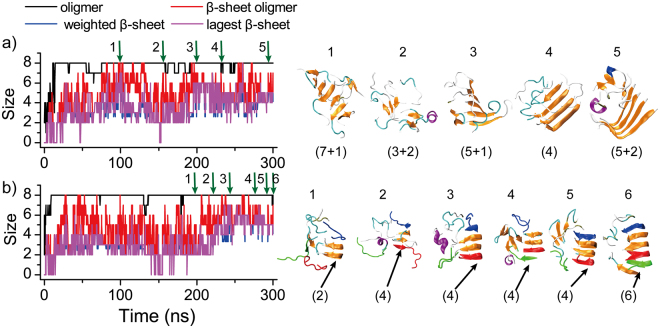
Figure 4.
The aggregation dynamics of hIAPP15–25. The aggregation processes of eight peptides from isolated random coil conformations to single-layer β-sheets with either L-turn (a) and U-turn (b) morphologies. The largest oligomer size (black), the largest β-sheet oligomer size (red), the mass-weighted average β-sheet size (blue) and the largest β-sheet size (purple) were plotted as the function of simulation time. The snapshot structures along the simulation trajectories as pointed by green arrows were given on the right lane, where each peptide was shown in cartoon representation with strand colored in yellow, coil in gray, helix in purple, and turn in cyan. The sizes of β-sheets were given in the parentheses. In panel b, three initially unstructured peptides (colored in red, blue and green, respectively) were highlighted to illustrate their binding, structural rearrangement, and eventually incorporation into the final β-sheets.