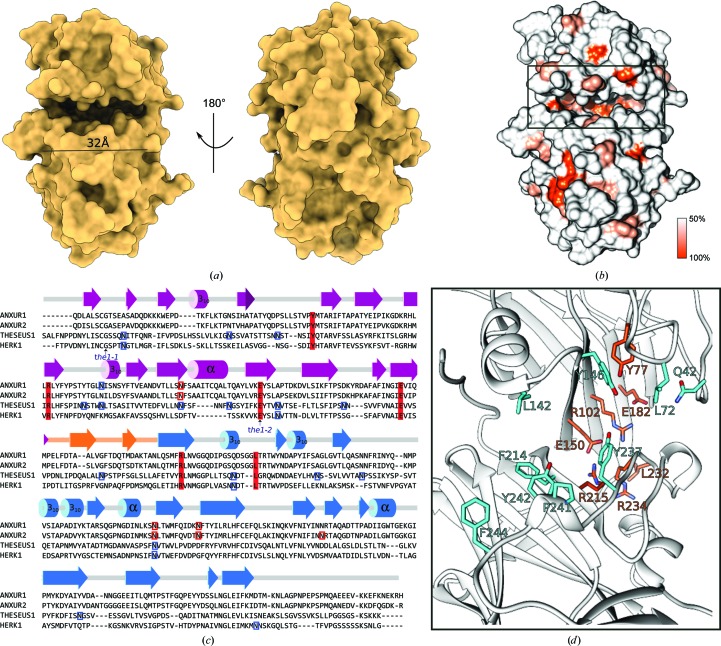
Figure 5.
Plant tandem malectin-like receptor kinases feature a unique ligand-binding cleft. (a) Front and back views of the ANX1 ectodomain in surface representation reveal the presence of a wide and deep cleft located at the interface between the N- and C-terminal malectin-like domains. (b) Surface representation of ANX1 coloured according to CrRLK1L family sequence conservation. (c) Sequence alignment with secondary-structure assignment for ANX1 calculated with DSSP (Touw et al., 2015 ▸) and coloured according to Fig. 1 ▸. Predicted and experimentally verified N-glycosylation sites are highlighted in blue and red, respectively. The known genetic THE1 missense alleles are indicated by an arrow and the two conserved cysteine residues in CrRLK1Ls are highlighted by light orange boxes. (d) Close-up view of ANX1 (as a ribbon diagram) with conserved interface residues highlighted in orange and with selected apolar and aromatic cleft-lining residues depicted in cyan (in bond representation). Residue identifiers are according to the ANX1 sequence. The depicted residues are highlighted in (c) using the same colour code.