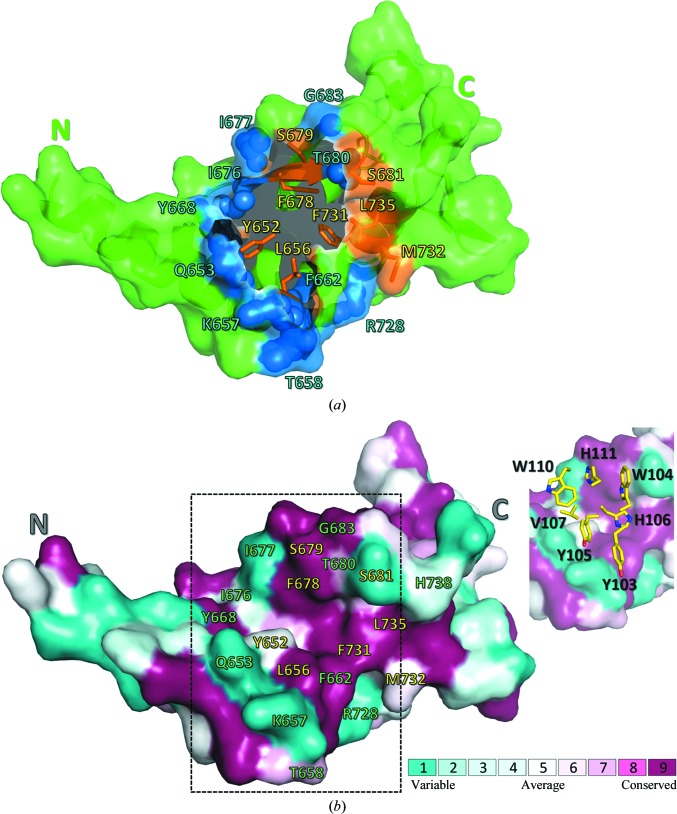
Figure 4.
The topology and evolutionary conservation of the BDBV NPCt surface involved in the interaction with MJ20. (a) BDBV NPCt is represented schematically by a green ribbon, with the solvent-accessible surface (within the complex with MJ20) shown in a semitransparent manner. The N- and C-termini are marked for clarity. The MJ20-binding concave crevice is located centrally between the N-terminal α-helix hairpin and the C-terminal lobe of the protein. The residues forming the largest contacts with MJ20HC are represented by blue spheres, while eight conserved residues targeted by alanine scanning (see text) are shown as orange sticks. (b) The same view of the BDBV NPCt surface, showing amino-acid conservation among the five strains of the virus. The MJ20 interface (delineated by dashed lines) is shown again in the inset, with a stick representation of the critical seven amino acids from the CDR3HC loop of MJ20.