Figure 1. Study Schema.
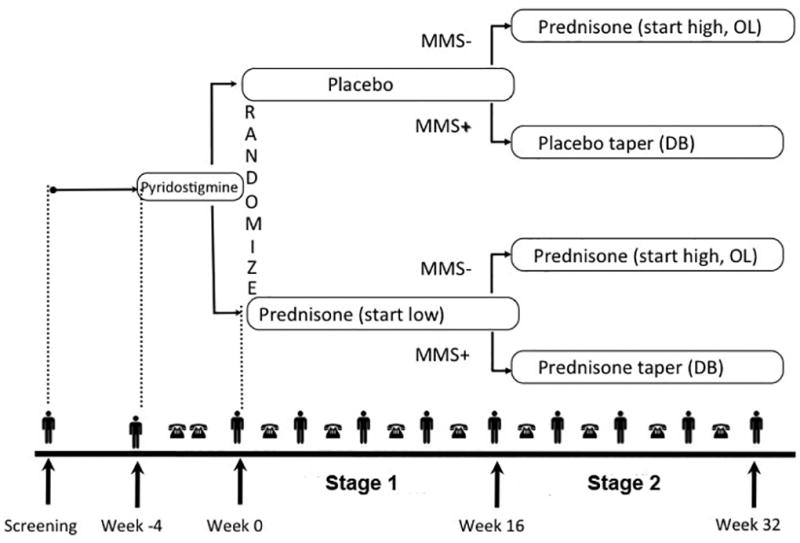
Following enrollment (Week -4), study participants were treated with escalating doses of pyridostigmine for a period of 4 weeks. Those who did not achieve MMS were randomized to receive prednisone or placebo in a double-blind manner (Stage 1). Outcomes were assessed at Week 16, following which those participants who had not yet achieved MMS were crossed over to receive open-label high-dose prednisone (Stage 2). MMS – minimal manifestation status; OL – open label; DB – double-blind.
 indicates in-person visit;
indicates in-person visit;
 indicates telephone visit.
indicates telephone visit.
