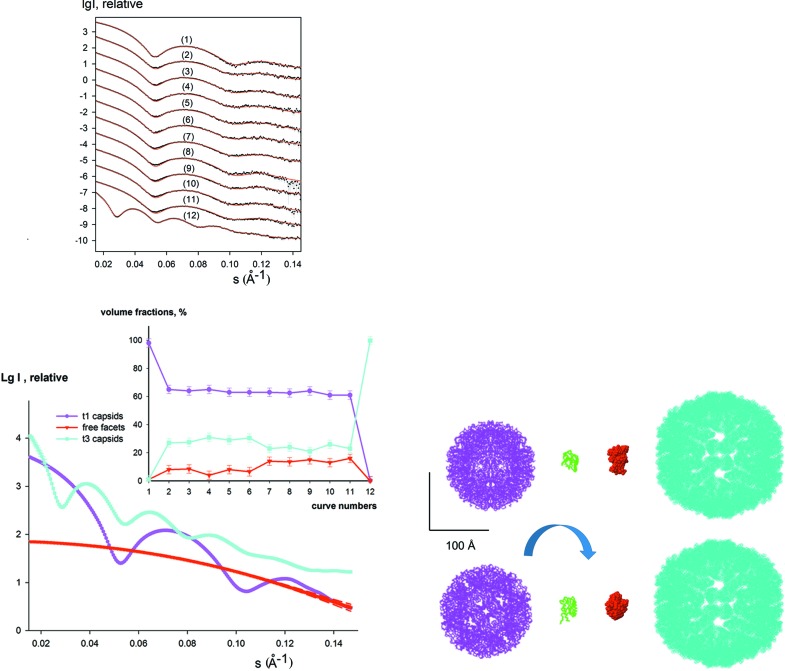
Figure 3.
Intermediate detection in lumazine synthase capsid formation. The top left panel displays the data measured at different conditions [curve 1, wild type lumazine synthase from B. subtilis (LSBS) in borate buffer (pH 7.0); curves 2–6, wild type lumazine synthase from A. aeolicus (LSAQ) in phosphate buffer (pH 6.0, 6.5, 7.0, 7.5, 8.0); curves 7–11, LSAQ in Tris buffer (pH 7.0, 7.5, 8.0, 8.5, 9.0); curve 12, LSAQ mutant in Tris buffer (pH 8.0)]. The experimental data are shown as dots with error bars and the DAMMIX fits as red solid lines. Known ‘initial’ and ‘final’ states, t1 capsids of diameter 160 Å and t3 capsids of diameter of 300 Å, respectively, are shown in the bottom right panel with magenta and cyan beads. The experimental data from LSBS in borate buffer and LSAQ mutant in Tris buffer (curves 1 and 12, respectively) corresponding to these models were used as input in DAMMIX after regularization by GNOM (Svergun, 1992 ▸). The monomeric lumazine synthase (PDB entry 1rvv) is shown with green Cα traces, and a typical restored shape of the unknown component (dissociated fragments of capsids) obtained by DAMMIX is displayed with red beads. The scale bar is 100 Å. The scattering curves from the components are shown in the bottom left panel (the two most different restored curves for the intermediate obtained from multiple DAMMIX runs are shown with dashed red lines) and their restored volume fractions are displayed in the inset; the colours are the same as the bottom right panel and the error bars of volume fractions display the average dispersion over multiple DAMMIX runs.