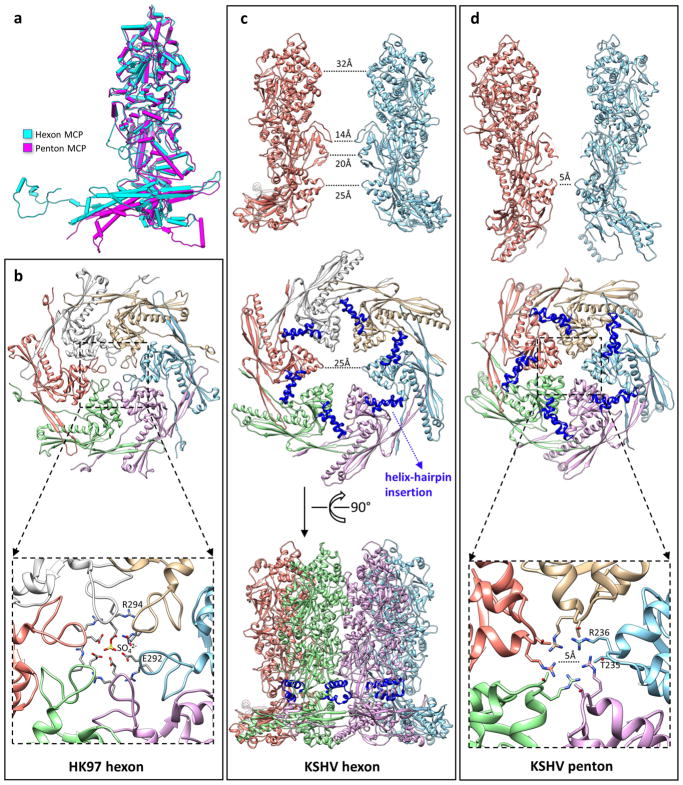
Extended Data Figure 6. Structural differences between hexon and penton.
a, Superimposed models of a hexon MCP and a penton MCP, which shows the hinged tilting of the floor region of a penton MCP towards the centre of the capsid. b–d, Comparison of channel constrictions between a bacteriophage HK97 hexon (b), a KSHV hexon (c) and a KSHV penton (d). The hexon channel in HK97 is tightly constricted by a loop in the A-domain (Fig. 2k) of the Johnson fold (b, top). In the crystal structure of HK97, this channel is completely blocked by a sulfate ion53 (b, bottom). The hexon channel in KSHV is not constricted by the HK97-like Johnson fold. The diameter of the channel at this position is 25 Å (c, middle). Note the large gap between adjacent Johnson folds, and also the absence of a long loop corresponding to the channel-constricting loop in HK97. Instead, the KSHV hexon channel is most constricted at the channel domain (c, top). Side view of the hexon (c, bottom) shows that the helix-hairpin domain insertion (blue) seals a hole at the root of the capsomer tower. The penton channel in KSHV is constricted by the Johnson-fold domain (d, middle). The diameter of the penton channel at this position is 5 Å (d, bottom). Owing to the hinged tilting of the floor region of penton MCP towards the centre of the capsid (a), adjacent Johnson-fold domains move closer to one another and constrict the penton channel (d, top).