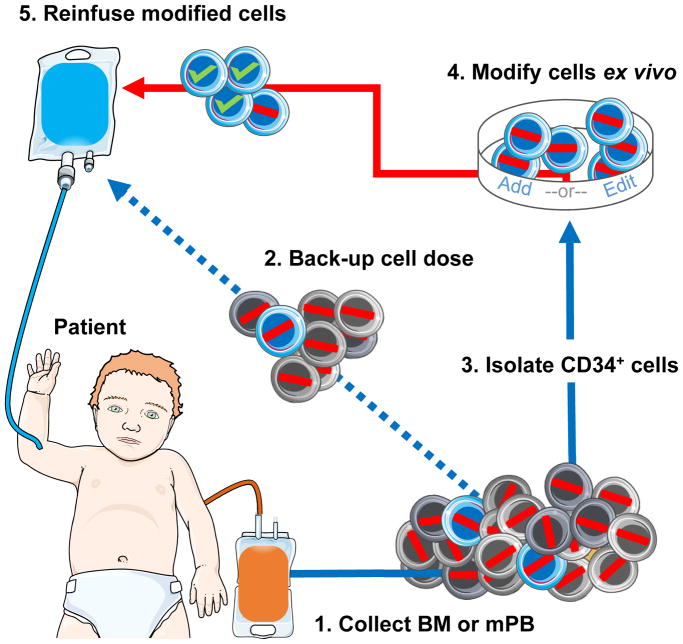
Figure 2. Autologous hematopoietic stem cell transplantation combined with gene addition or editing.
(1) Bone marrow (BM) or mobilized peripheral blood (mPB) cells are collected from the patient (red line represents a disease-causing mutation). Typically, 15–20ml of BM/Kg is an acceptable harvest target. While collecting HSCs by mobilization and apheresis is less invasive than BM aspiration, infants have small blood volumes making leukapheresis challenging. Failure to harvest adequate cell numbers can prevent therapy. (2) Modification of HSCs may reduce stem cell capacity. A back-up cell dose of non-modified cells is apportioned to restore native hematopoiesis in the event of graft failure. (3) CD34+ cells are isolated in a GMP-compliant, closed system. Purification of HSCs may reduce total cell number as CD34+ HSCs represent less than one percent of total cells. Alternatively, a CD34+/CD38− enrichment strategy may be employed to further purify HSCs and lower the amount vector required for modification. CD34+ cells may be pre-stimulated ex vivo for 1–3 days prior to modification, depending on the protocol. (4) Gene modification of HSCs must be permanent so as to be passed down to all progeny. Cells are modified by either a viral vector to add a gene (typically requires high concentration vector), or targeted nucleases with/without a donor template to disrupt, correct, or insert a gene. After ex vivo modification, the cell product undergoes release testing to assess purity, identity, safety, potency (transduction/editing efficiency), and other characteristics. If the modification strategy requires selection of corrected cells, low cell yield may prevent transplantation. (5) Prior to receiving the cell product, the patient undergoes conditioning to “make space” for engraftment of modified HSCs (green check represents successful modification of a disease-causing gene). Modified cells may be reinfused fresh or cryopreserved for delivery at a later time. While high-levels of cytoreductive agents may be toxic, inadequate conditioning may result in poor engraftment.