Table 1.
Optimization of the Reaction Conditionsa
| |||||
|---|---|---|---|---|---|
| entry | acid / base | oxidant | atmosphere | solvent | yieldb |
| 1 | KHCO3 | DMBQ | air | HFIP | (59) |
| 2 | KHCO3 | benzoquinone | air | HFIP | (34) |
| 3 | KHCO3 | dimethoxybenzoquinone | air | HFIP | (7) |
| 4 | AcOH | DMBQ | air | HFIP | n.d. |
| 5c | KHCO3 | DMBQ | air | HFIP | (40) |
| 6 | K2HPO4 | DMBQ | air | HFIP | (42) |
| 7 | iPr2NEt | DMBQ | air | HFIP | (30) |
| 8 | KHCO3 | DMBQ | air | tAmylOH | (10) |
| 9 | KHCO3 | DMBQ | air | DMF | (5) |
| 10 | KHCO3 | DMBQ | air | dioxane | (10) |
| 11d | KHCO3 | DMBQ | air | HFIP | (12) |
| 12e | KHCO3 | DMBQ | air | HFIP | (14) |
| 13f | KHCO3 | DMBQ | air | HFIP | (23) |
| 14 | KHCO3 | DMBQ | N2 | HFIP | n.d. |
| 15 | KHCO3 | DMBQ | O2 | HFIP | 65 |
| 16 | KHCO3 | DMBQ (0.5 equiv) | O2 | HFIP | 50 |
| 17 | KHCO3 | DMBQ (2.0 equiv) | O2 | HFIP | 80 |
| 18 | KHCO3 | DMBQ (3.0 equiv) | O2 | HFIP | 73 |
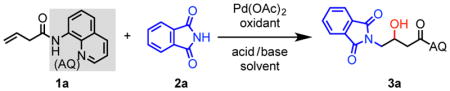
Reaction conditions: 1a (0.1 mmol), 2a (1.5 equiv), Pd(OAc)2 (10 mol %), oxidant (1.5 equiv), base (1 equiv), solvent (0.2 mL), 100 °C, 12–16 h.
Isolated yield. Values in parentheses represent yields determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-triisopropylbenzene as internal standard.
KHCO3 (0.5 equiv).
120 °C.
80 °C.
4Å molecular sieves as additive.
