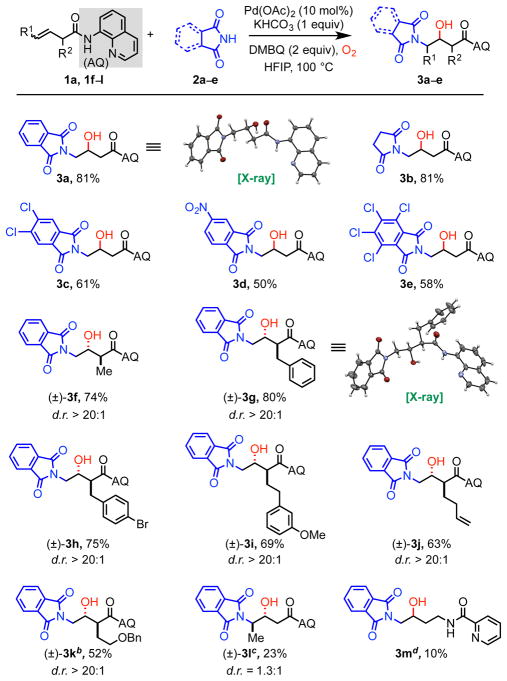
Table 2.
Reaction Substrate Scope.a
Reaction conditions: 1a (0.1 mmol), 2a (1.5 equiv), Pd(OAc)2 (10 mol %), DMBQ (2 equiv), K2CO3 (1 equiv), HFIP (0.2 mL), 100 °C, O2 (1 atm), 12–16 h. Percentages refer to isolated yields.
Intramolecular cyclized compound was also recovered (see footnote 13).
Pd(OAc)2 (15 mol%), 120 °C, 2 d.
N-(but-3-en-1-yl)picolinamide was used as the alkene.