Figure 4.
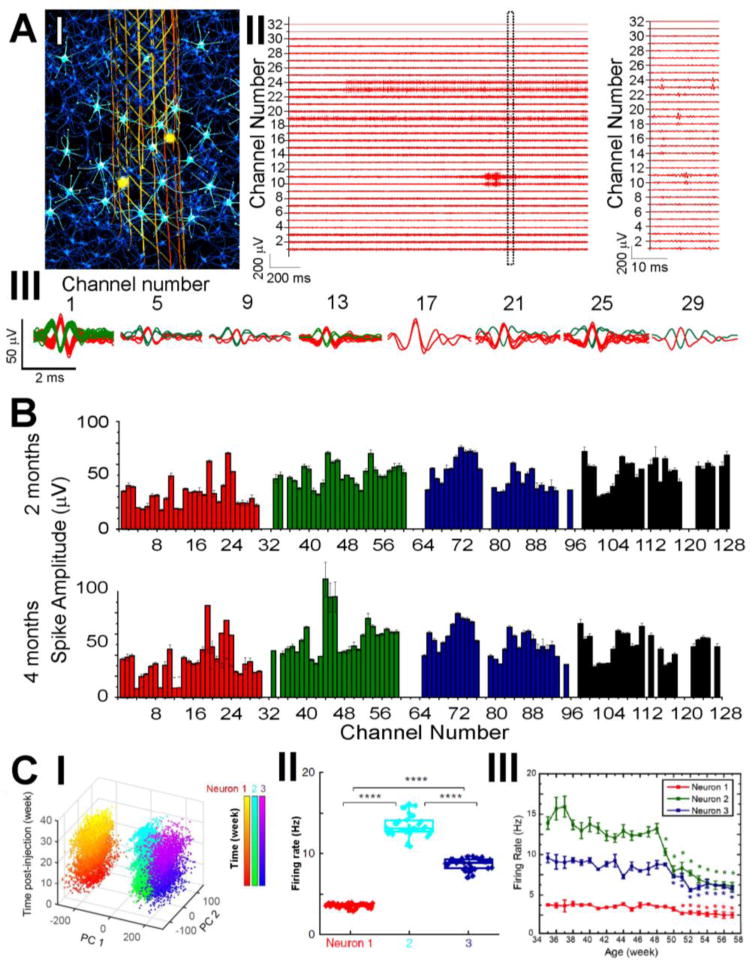
Highly multiplexed, chronically stable in vivo electrophysiology in mice. (A) Electrophysiological recording of single-neuron action potentials in mouse brain. I, Schematic of in vivo recording of neuron activities via the seamless integrated interface between the injected mesh electronics and the endogenous neural tissue. Electrically active neurons are highlighted in bright cyan color, with their firing activities detected by a subset of recording electrodes (yellow circles) in the mesh electronics at a time. II, Representative multiplexed extracellular single-neuron recording traces from a 32-channel mesh electronics probe at 2 months post-injection into the mouse motor cortex, with a magnified view of a 20-ms segment highlighting the waveforms of detected single-unit action potentials in Channels 10, 11, 19, 23 and 24. III, Overlay of sorted and clustered spikes from a subset of selected channels by processing the raw data of recording traces shown in A, II. Red and green colors are used to denote spikes assigned to different neurons. (B) Average single-unit spike amplitudes from simultaneous 128-channel recording in a mouse brain using 4 separate 32-channel mesh electronics probes at 2 and 4 months post-injection. The error bars represent the s.e.m. (C) Time-dependent consistent tracking of the same individual neurons based on single-unit spike recording, sorting and clustering as shown in B. I, PCA-separated clusters of single-unit firing activity are stable over the course of 34 weeks post-injection in young mice. II, PCA-identified neurons from C, I demonstrate stable firing rates across the analyzed 34 weeks. III, Age-dependent individual neuron firing rate changes in middle-aged mice demonstrate systematic decline. Reproduced with permission from refs. 32 and 33.
