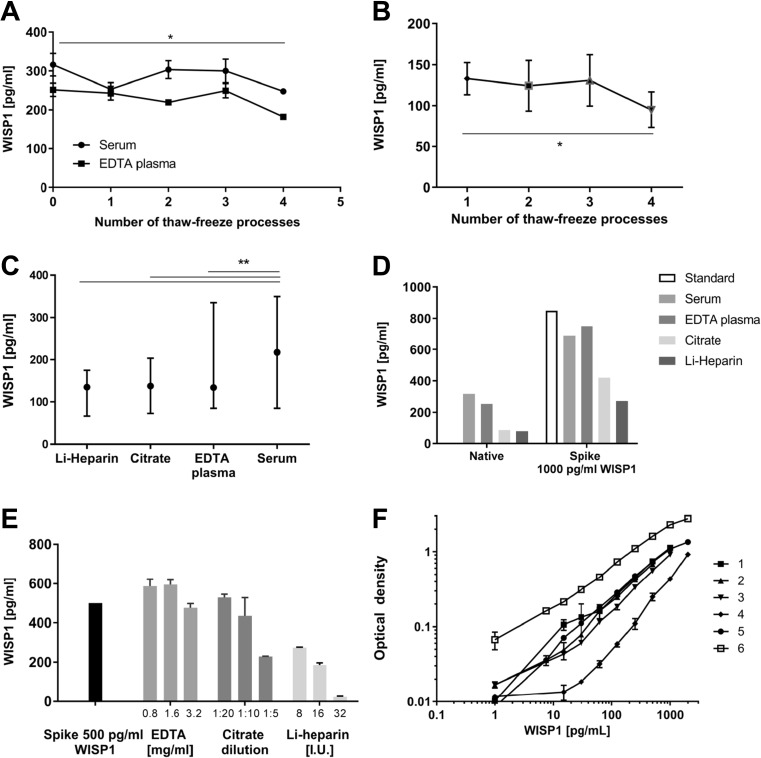
Fig. 1.
Validation of WISP-1/CCN4 assay. WISP-1/CCN4 concentrations were measured (a) after multiple freeze-thaw cycles in fresh collected and pooled serum and plasma samples (n = 9); (b) after multiple freeze-thaw cycles in pooled serum samples from cohort III (n = 8); (c) in serum, EDTA plasma, citrate plasma and Li-heparin plasma samples at the same freeze-thaw cycle (n = 14); (d) in pooled serum and plasma samples spiked with 1000 pg/mL of recombinant human WISP-1/CCN4 (data shown as median ± interquartile range); (e) in reagent diluent samples with different concentrations of clotting inhibitors and 500 pg/mL of recombinant human WISP-1/CCN4. (f) Example figure of standard curves in the human WISP-1/CCN4 DuoSet ELISA kit (DY1627) assay. *p < 0.05, ** p < 0.01