Figure 2.
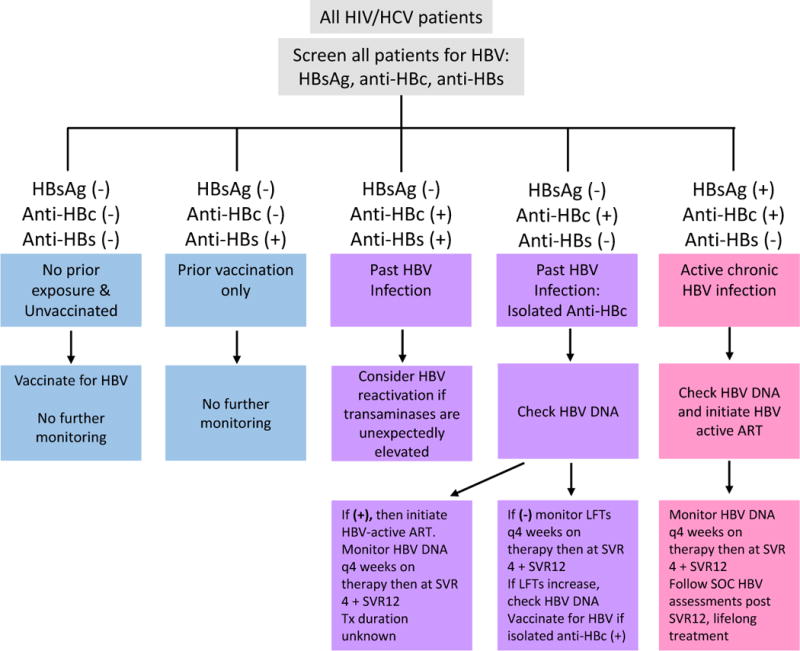
HBsAg: HBV surface antigen; Anti-HBc: antibody to HBV core antigen; Anti-HBs: antibody to HBsAg; HBV-active ART: Regimens containing TDF, TAF + 3TC; entecavir should not be used in patients with HIV viremia; SVR4: Undetectable HCV RNA 4 weeks after end of therapy; SVR12: Undetectable HCV RNA 4 weeks after end of therapy; LFTs: liver function tests; SOC: standard of care
Modified from: Jacinta A. Holmes, Ming-Lung Yu & Raymond T. Chung (2017) Hepatitis B reactivation during or after direct acting antiviral therapy – implication for susceptible individuals, Expert Opinion on Drug Safety, 16:6, 651-672, DOI: 10.1080/14740338.2017.1325869
