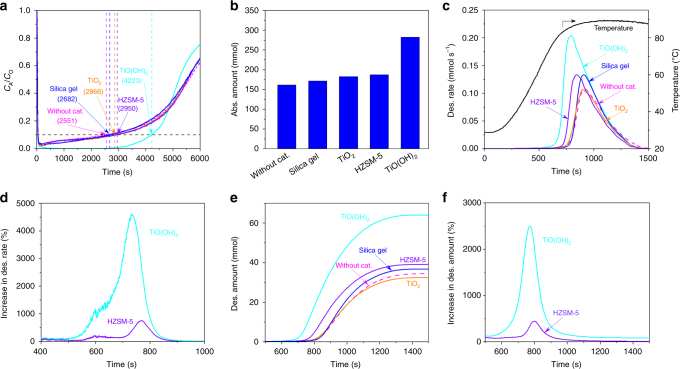
Fig. 1.
Effect of TiO(OH)2 catalyst on CO2 absorption (abs.) and desorption (des.). a Uncatalyzed and catalyzed CO2 absorption profiles of 20 wt% MEA solution. b The quantities of the CO2 sorbed within the effective absorption time (>90% CO2 capture). c The rates of CO2 desorption from spent 20 wt% MEA sorbent without and with uses of catalyst (cat.). d The percentage increases in CO2 desorption rate due to the use of TiO(OH)2. e Effects of TiO(OH)2 on the quantities of desorbed CO2. f The percentage increases in CO2 desorption amount due to the use of TiO(OH)2. Absorption conditions—total mass of solution: 200 g; MEA concentration in the solution: 20 wt%; total flow rate of gas: 1000 mL/min; composition of gas: 10 vol% CO2, 10 vol% O2, and 80 vol% N2; temperature: 25 °C; absorption time: 6000 s. Desorption conditions—Total mass of solution: 200 g; MEA concentration in the solution: 20 wt%; temperature: 88 °C; time: 2400 s