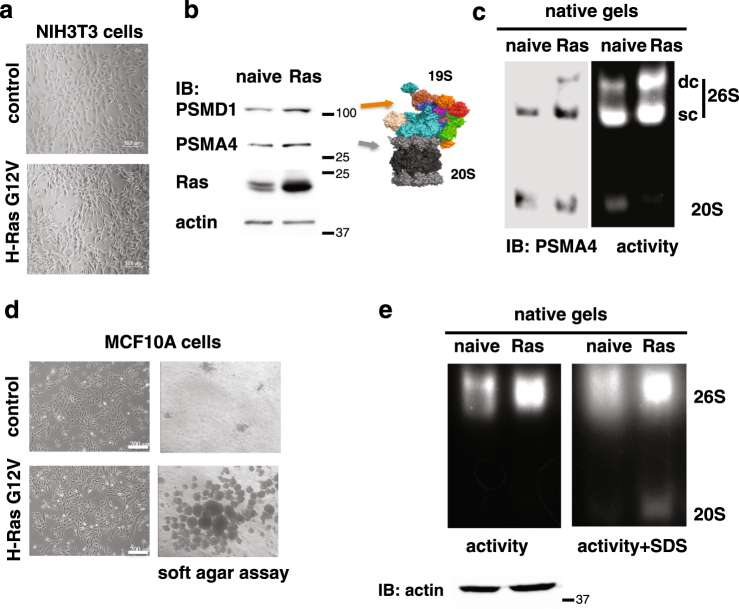
Fig. 1. The H-Ras G12V-transformed NIH3T3 and MCF10A cells contain high levels of 26S proteasome.
a NIH3T3 cells were transduced with retroviruses carrying H-Ras G12V gene, and visualized by microscopy. The cells displayed a transformed phenotype with a spindle-shaped and highly refractile morphology. b Expression of the proteasomal subunits PSMA4, a 20S component, and PSMD1, a 19S component, was tested by immunoblot (IB). c 26S and 20S proteasomal complex activity and levels were analyzed by native gel electrophoresis in both naive NIH3T3 and Ras-transformed cells. Equal amount of total protein, determined by Bradford assay, was loaded onto native gels (actin loading control is represented). The 26S complex is either double capped, namely each of both ends of the 20S proteasome is occupied by a 19S complex (DC-26S) or single capped (SC-26S). d MCF10A were transduced with retroviruses carrying pBabe H-Ras G12V gene, visualized by microscopy and subjected to soft agar assay. Transformation was evidenced by a fibroblast-like appearance and a dispersed cell distribution in monolayer culture (left) and by anchorage-independent growth in soft agar (right). e 26S and 20S proteasomal complex activity and levels were analyzed by native gel electrophoresis in both naive MCF10A and Ras-transformed cells. In the right panel, for the visualization of the 20S complex, the proteasomal activity was enhanced by the addition of 0.02% SDS to the activity reaction