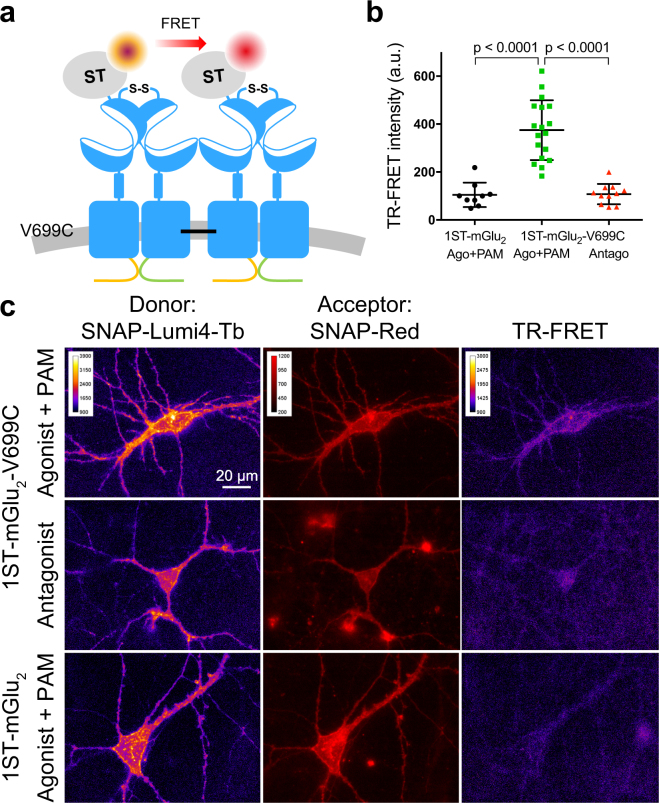
Figure 5.
mGlu2 dimers are in close physical proximity in the active state. LRET between mGlu2 dimers with a cysteine introduced in TM4 (V699C) that can form disulfide bonds between dimers upon incubation with the oxidizing agent copper phenanthroline (CuP) when the receptor is in the active state. (a) Schematic drawing of the experimental set up. A quality control system based on modified GABAB C-terminal domains was used to ensure that each mGlu2 dimer only contained one SNAP-tag (1ST-mGlu2). LRET was measured between SNAP-Lumi4-Tb (donor) and SNAP-Red (acceptor) covalently attached to the SNAP-tags. (b) Scatter plot of the TR-FRET intensity in the low expression range (dashed lines in Supplementary Fig. S6). In this interval the TR-FRET intensity for 1ST-mGlu2-V699C incubated with CuP in presence of agonist and PAM (10 nM LY379268 and 3 μM BINA) is significantly higher than when the same receptor mutant is incubated with CuP in presence of antagonist (400 nM LY341495) or the negative control 1ST-mGlu2 incubated with CuP in presence of agonist and PAM (p < 0.0001, one-way ANOVA, Bonferroni’s multiple comparisons test). Data are from 4 (1ST-mGlu2-V699C+ agonist and PAM) or 2 (controls) independent experiments. (c) Representative donor, acceptor and TR-FRET images of 1ST-mGlu2-V699C incubated with CuP in presence of either agonist and PAM or antagonist, and 1ST-mGlu2 incubated with CuP in presence of agonist and PAM.