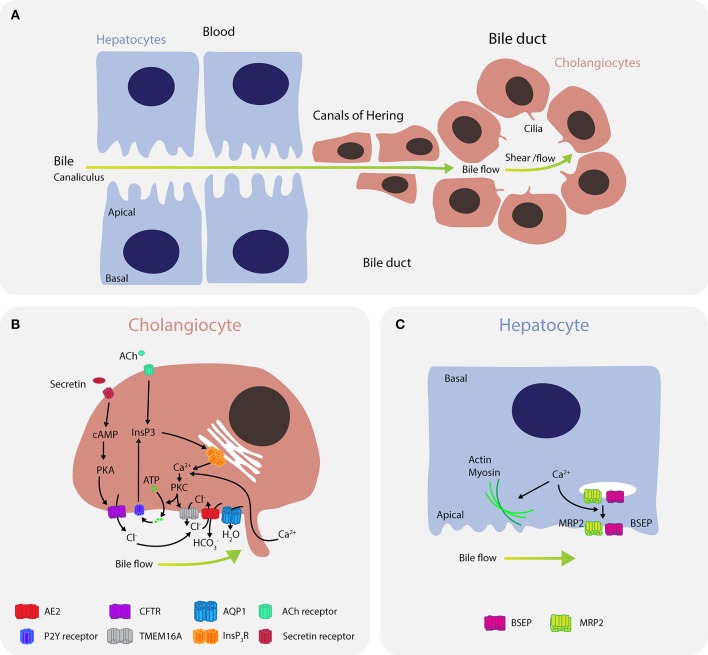
Figure 4.
Calcium signaling regulates bile flow in hepatocytes and cholangiocytes. (A) Bile is secreted by hepatocytes into the canaliculus where it is transported to canals of Hering and then into bile ducts. (B) In cholangiocytes, calcium is predominantly released through the type III isoform InsP3R channels (ITPR3) in response to signaling via M3 muscarinic acetylcholine receptors (CHMR3) and purinergic receptors P2. Furthermore, mechanical cues, such as bile flow and shear stress, induce calcium uptake from the bile through ion channels in the mechanosensory primary cilium. Bicarbonate () exchange through the anion exchange protein 2 (AE2) depends on extracellular chloride concentrations (Cl−), which are regulated by TMEM16A and CFTR. Calcium activates the chloride channel TMEM16A to mediate chloride-bicarbonate exchange at the apical side, while CFTR signaling is activated when secretin binds the secretin receptor on the basolateral membrane, leading to formation of cAMP, activation of PKA and efflux of chloride through the phosphorylated CFTR. Downregulation of ITPR3 in cholestasis, which severely disrupts calcium signaling in cholangiocytes, is thought to be a key mechanism determining bile flow and pathology. Dysregulation of calcium signaling affects multiple pathways regulating both secretion and bile flow. (C) In hepatocytes, calcium is affected by ATP, angiotensin II, vasopressin, glucagon, or epinephrine, and regulates actin-myosin contractility to control peristaltic contractions, as well as exocytic insertion of the bile salt export pump (BSEP) and the multidrug resistance-associated protein 2 (MRP2).