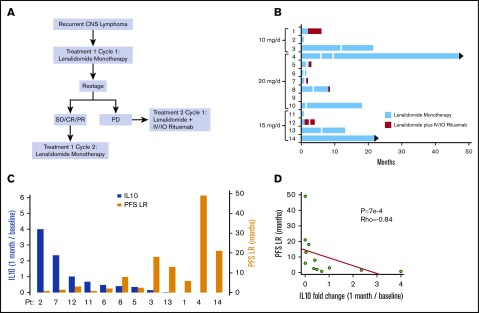
Figure 1.
Phase 1 design and response duration to lenalidomide, lenalidomide/rituximab, and CSF IL-10 as a pharmacodynamic biomarker of response. (A) Protocol schema. (B) Swimmer plot of response duration to lenalidomide monotherapy (treatment 1) and to lenalidomide plus rituximab (treatment 2). Y-axis, study subjects and assigned dose level; x-axis, months on protocol; vertical white lines, dose reduction of lenalidomide. Only 3 of the 9 patients that responded to lenalidomide monotherapy received concomitant dexamethasone. Median OS for the 14 patients is 15.5 months. (C) Y-axis (left in blue), change in CSF IL-10 at 1 month compared with baseline; y-axis (right in yellow), PFS on lenalidomide plus rituximab, in months. There was an inverse correlation between the magnitude of change of IL-10 in CSF at 1 month restaging compared with baseline with response duration to lenalidomide. CSF concentration of IL-10 was determined at pretreatment baseline by enzyme-linked immunosorbent assay (Becton Dickenson) and at 1 month restaging. IL-10 was detected in pretreatment CSF specimens in 13 of 14 patients (92.8%). (CSF IL-10 was not detected at baseline in patient 10, who had CNS DLBCL transformed from CLL.) (D) The inverse correlation between magnitude of change of IL-10 in CSF with response duration to lenalidomide is significant, as demonstrated by Spearman correlation P < .0007 and ρ = −0.84. IO, Intra-Ommaya; LR, lenalidomide/rituximab; OS, overall survival; PFS, progression-free survival; PD, progressive disease; PR, partial response; SD, stable disease.