Fig. 1.
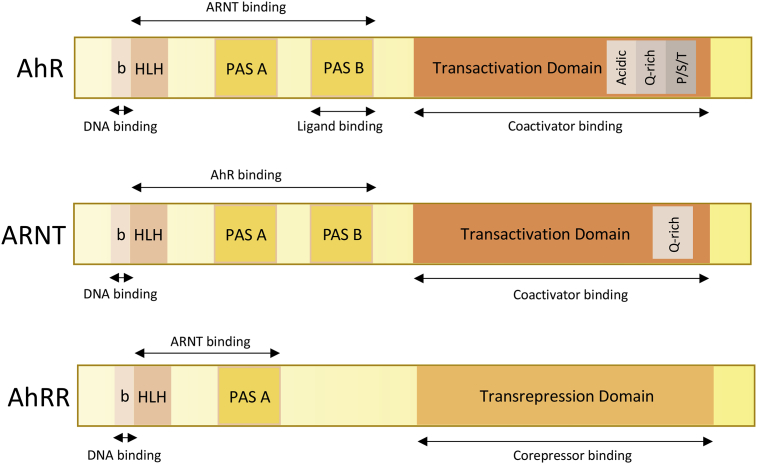
The functional domains of the AhR, ARNT and AhRR proteins. The AhR contains 1) a bHLH domain that allows the dimerization with its partner ARNT, the binding of DNA and the interactions with chaperones such as Hsp90 (Heat Shock Protein 90); it also contains sequences important for both nuclear import and export [76]; 2) a PAS domain which comprises two structural repeats A and B which are also involved in the dimerization with ARNT (PAS A) but which also allows the ligand binding (PAS B); 3) a C-terminal domain which contains three subdomains: one subdomain which is enriched with acidic residues (glutamate/aspartate), another one which is enriched with glutamine (Q-rich) and a third one which is enriched with serine, threonine & proline (S/T/P). Coactivators and co-repressors interact with the AhR via this domain [77], [78]. ARNT has a structure similar to AhR: The bHLH and PAS A domains are involved in the dimerization with AhR or AhRR and in DNA-binding. But in spite of the presence of a PAS B domain, ARNT is not able to bind ligands. AhRR also contains a DNA-binding domain (bHLH) and a dimerization domain (PAS A). The absence of the PAS B domain leads to its inability to bind ligands [79].