Fig. 3.
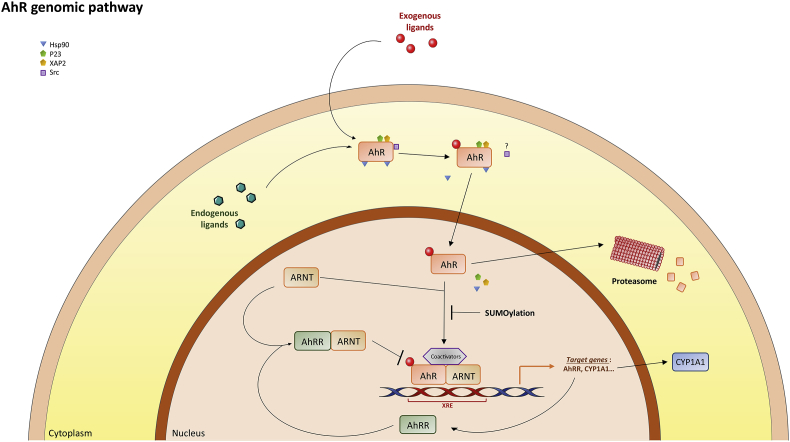
The AhR genomic pathway. When a ligand crosses the plasma membrane (passive diffusion), it binds to the AhR (the PAS B domain). This allows the translocation of the ligand-receptor complex into the nucleus and the dissociation of the receptor complex. In the nucleus, the complex heterodimerizes with its partner ARNT (AhR Nuclear Translocator also called HIF-1β) [90]. The heterodimer binds specific DNA sequences located in the promoter regions of target genes named xenobiotic response elements (XRE, 5′-TA/TGCGTG-3′) [80]. AhR-ARNT induces the transcription of target genes by the recruitment of various components of the transcriptional machinery [91] such as CBP/p300 (cAMP response element-binding protein binding protein) [80], [81], [82], SRC-1 (steroid receptor coactivator 1), p160/bHLH-PAS, NCoA2/GRIP1/TIF2 (Nuclear receptor Coactivator 2/Glucocorticoid Receptor Interacting Protein 1/Transcriptional Intermediate Factor 2) and p/CIP (p300/CBP/CoIntegrator-associated Protein) [95] as well as other transcriptional coactivators such as RIP140 (Receptor Interacting Protein 140) [96], [97] or ATP-dependent chromatin remodeling components such as BRG-1 (Brahma Related Gene). After being exported out of the nucleus, the AhR is rapidly degraded in the cytoplasmic compartment by the proteasome [12]. Other post-translational modifications such as SUMOylation of the human AhR have been observed [13].