Abstract
The small intestinal villus and its associated epithelium includes enterocytes as the main cell type and differentiated goblet and argentaffin cells, while the invaginated crypt epithelium is the site of cell division and hence the origin of all epithelial components. Enterocytes form a cohesive monolayer which acts both as a permeability barrier between lumen and the interior, and an important gateway for nutrient digestion, absorption and transport. Differentiation and polarisation of enterocytes depends on cytoskeletal proteins that control cell shape and maintain functionally specialised membrane domains; extracellular matrix (ECM) receptors; channels and transporters regulating ion/solute transfer across the cell. The mesenchymally-derived basement membrane dynamically controls morphogenesis, cell differentiation and polarity, while also providing the structural basis for villi, crypts and the microvasculature of the lamina propria so that tissue morphology, crucially, is preserved in the absence of epithelium. Mucosal re-organisation requires immense cooperation between all elements within the lamina, including marked revisions of the microvasculature and extensive alterations to all basement membranes providing support for endodermal and mesenchymal components. In this context, subepithelial myofibroblasts fulfil important regulatory activities in terms of tissue morphogenesis; remodelling; control of epithelial cell development, polarity and functional attributes; and an intimate involvement in repair, inflammation and fibrosis.
This paper reviews the main structural and functional aspects of the villus, including the epithelium and its outer glycocalyx and microvillous border; and subjacent to the epithelium, the basement membrane with its attached web of myo-fibroblasts together with the lamina propria core of the villi, and its microvasculature and lacteals. Finally, some comments on the rapidity with which the overall structure of the villi changes in their response to both external, and internal, influences.
Key Words: Small intestinal villus, Permeability, Epithelium
Introduction–Why "Villus"?
Although the Latin "villus" refers to the "shaggy haired" nature of animals coats, Gabriele Falloppio (1523-1562), of tubal fame, first used the word "villi" in his 16th Century text "Observationes Anatomicae" (1561). However, his description derived from the feel and texture of velvet (1): any connection with the Latin is thus obscure.
During the 17-18th Centuries, microscopic anatomy did not exist, so that intestinal "villi" were deemed analogous to dermal papillae (rete pegs), since the overlying cellular basis of either dermis and epithelium – yet to be discovered - was simply regarded as an amorphous, gelatinous surface coating. One can therefore understand why X. Bichat (1771-1802) came to employ the term "mucous membrane" for the first time in his "Traite des Membranes" (2) – although goblet cells had yet to be recognised. Thus intestinal "villi", at first, were only perceived in terms of their central cores, being seen as small, upwardly-projecting structures in wet preparations, their vessels defined through injections of red and green wax into mucosal arteries and veins.
The widespread use of microscopes during the 19th Century gave rise to the earliest descriptions of the cellular nature of the so-called "epithelium" (coined from the Greek ἐpi = upon, and θhlή = nipple). Two almost simultaneous sources for the discovery of epithelial cells came from F.G.J. Henle (of the renal tubular loop) in Germany (1837) (3), and W. Bowman (of renal capsular fame) at King's College, London (4). However, Bowman's predecessor, Robert Todd, revised Henle's use of "prismatic" to "columnar" in describing individual cells.
A modern account of the villus and its associated epithelium includes the differentiated goblet and argentaffin cells, while the invaginated crypt epithelium is the site of cell division and hence the origins of all epithelial components. This paper reviews the main structural and functional aspects of the villus, including the epithelium and its outer glycocalyx and microvillous border; and subjacent to the epithelium, the basement membrane with its attached web of myo-fibroblasts and cognate cells – pericytes, smooth muscle, fibroblasts and muscularis mucosae, and outer cells of Cajal. Here we encounter the lesser explored lamina propria core of the villi, which includes the microvasculature and lacteals. Finally, some comments on the rapidity with which the overall structure of the villi changes in their response to both external, and internal, influences.
The epithelium and brush border – the ins and outs
The intestinal tract is lined by a single layer of columnar epithelium originating from multipotent stem cells at the base of each crypt, giving rise (5) to four major types of epithelial cells: (i) absorptive enterocytes comprising >80% of all small intestinal epithelial cells; (ii) goblet cells producing various mucins and trefoil peptides needed for epithelial growth and repair; (iii) entero-endocrine cells which export peptide hormones; and (iv) Paneth cells which secrete antimicrobial cryptidins or defensins, digestive enzymes, and growth factors. Following their differentiation, enterocytes, goblet and entero-endocrine cells migrate upwards thus to be exfoliated (from presumptive "extrusion zones") (6) at the villous tips after approximately five days (7). Transfection of basal crypt cells with marker proteins permits examination of the successive changes occurring in enterocytes as they leave the crypts (8). Indeed, distinctive switches in gene expression patterns during contact with neighbouring cells casues down-regulation of differentiation signals in favour of those now necessary for specialised functions as polarised cells, especially at the brush border zone (9,10 ).
Complex signaling pathways implicated in the regulation of specific differentiation of the cells in the intestine include Wnt -β-catenin-TCF, Notch and its downstream effectors - HES1 and Math1, BMP-TGF-β-SMAD, and hedgehog (Hh). A number of transcription factors, many of which are downstream targets of these signaling pathways, including cdx-1 and cdx-2; kruppel-like factor; GATA4, 5 and 6 together with several forkhead family members, E-cadherin-mediated cell-cell and integrin-mediated cell-matrix adhesion; chemotactic gradients; extracellular matrix and mesenchymal components; and a range of cytokines, hormones and growth factors, have each been implicated in the regulation of intestinal cell maturation. This is too expansivea field for detailed discussion here.
As newly-formed enterocytes migrate from the crypts, they develop the apical "brush border" comprising microvilli approximately 1μM in length and 0.1μM in diameter. There are approximately 3,6000 (±450) microvilli per cell (Marsh, unpublished) thereby increasing the surface area by ~10-20 fold (11). The resulting increased 'reserve' of surface membrane allows additional specialised functions permitting membrane-associated macromolecular digeston and absorption (12,13), and facilitating defences at this important host-environmental interface (14).
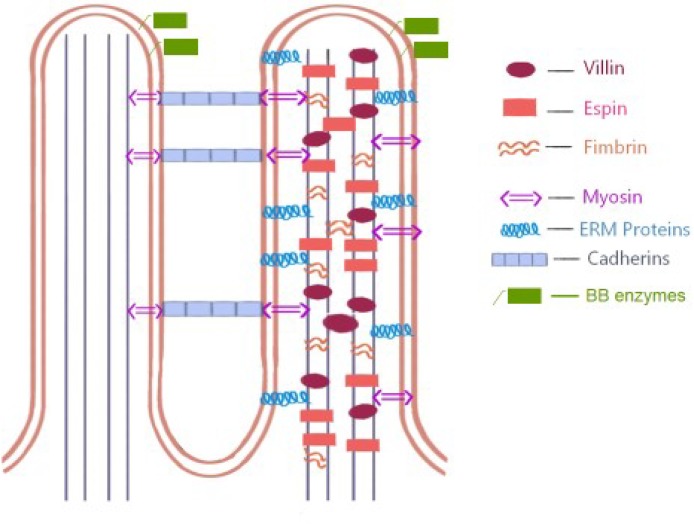
Together, enterocytes form a cohesive monolayer which acts as a permeability barrier between lumen and the interior, and as an important gateway for nutrient digestion, absorption and transport (15). Differentiation and polarisation of enterocytes depends on cytoskeletal proteins (16,17) that control cell shape and maintain functionally specialised membrane domains; extracellular matrix (ECM) receptors; channels and transporters regulating ion/solute transfer across the cell (Figure 1). As already hinted, the microvilli together with their enzymes and transporter proteins represent the most important functional differentiation within the villus for digestion/absorption of carbohydrate, proteins, lipids, minerals and vitamins. Subsequent processing within the enterocyte requires the help of secretory and sorting pathways.
Figure 1.
This diagram represents the molecular structure of the BB. Actin filaments within each microvillus are bundled by villin, espin, and fimbrin which also serve to stabilize the actin core. Molecules such as unconventional myosins and ERM (ezrin, radixin, moesin) family proteins cross-link the plasma membrane to the underlying actin cytoskeleton while extracellular adhesion molecules such as cadherin family members—protocadherin-24 (PCDH24) and mucin-like protocadherin (MLPCDH) mediate intermicrovillar adhesion during brush border assembly. Between the external and internal surfaces of the microvillus membrane brush border enzymes are located. Adapted from Crawley et al (12).
The basement membrane – potentiating potential
Histologically, basement membranes appear as insignificant thin strips of homogeneous, amorphous pink material lying between epithelium and subjacent mesenchymal elements, such as myofibroblasts. Many years ago, this region was shown to be periodic acid- Schiff positive and hence rich in glycoprotein(s), while electron microscopy revealed the presence of fibrillar material.
Basement membranes are ubiquitous throughout the body, containing laminin-1, fibronectin, type IV collagen, entactin (nidogen), together with perlecan (18) as the major component of heparan sulphate proteoglycans (HSPG) (19). Laminin and collagen IV are mainly synthesised by mesenchyme, while HSPG derives from the basal epithelium, as also entactin (nidogen) (20). However, fully-formed and functional basement membrane depends critically on mesenchymal derivatives. Moreover, laminin A chain synthesis is accelerated, and selectively, as the foetal intestine matures towards the end of gestation with the onset of epithelial cell differentiations (21,22). There is also evidence of spatial orientation of HSPG and laminin chains more-or-less orthogonally across the basement membrane, thus not only strengthening the intermolecular cross-binding of these cruciate molecules (23), but also providing specific anchorage between them and overlying cell membranes. Their molecular dimensions indicate their capability of spanning the entire thickness of basal laminae (24,25 ).
Yet despite these recent insights, several problems remains (26): are basement membranes homogeneous throughout, both along the entire intestinal tract and at all levels between the crypts and villous tips? what relationship exists between the upward migration of the epithelium and possible molecular changes in the membrane at the so-called extrusion zones at tips of villi? and are cells only extruded from the upper reaches of the villi given, for example, the vast excess of crypts in some species? (27). These anatomical variants both in crypt ratios to villi and the structural variations between pencil-shaped and leaf-shaped villi, call into question the often-assumed existence of extrusion zones located (only) at villous tips. Very careful studies on resected pieces of full thickness bowel have been disappointing in revealing site(s) of desquamation (28), while the locus of the increased rate of enterocyte loss in flat coeliac mucosae, for example, has never been precisely defined. Indeed, the concept of "extrusion zones" at villous tips seems rather more a mythology than reality, perhaps. Rather, it is more likely that cells are removed at all points along the villous epithelium (29) even from the inter-villous regions of the mucosa (30) and may be the reason why desquamated cells are very difficult to observe, especially when only thin histological sections are employed for identification.
Another problem is how the epithelium, collectively, moves along the basement membrane. Earlier studies suggested that epithelium and subjacent myofibroblasts move upwards in a cohesive manner (31,32) but that may not be so (33): indeed, we have very little knowledge about the turnover of these cells and their means of replacement. We must also remember that due to villous tip narrowing, the changing apical geometry demands considerable shedding if the cells remaining are to retain contact with the basement membrane. That difficulty remains to be elucidated.
The appearance of the basement membrane as a continuous sheet is probably artefactual on account of the coagulation of its glycoprotein elements during fixation and processing: but scanning EM has drawn attention to "holes" (~0.5-5μM diameter) disrupting its continuity (34,35). It hardly seems likely that this membrane arises de novo as a perforated sieve. Rather, these perforations must therefore reflect those items, like migratory cells, basally-extruded enterocyte projections, together with inert material, which pass through it. It was shown in earlier studies that the membrane could be breached, albeit with some apparent mechanical difficulty. In those studies of inflammatory exudates, Sir Howard Florey in Oxford noted membrane bulging in the presence of emigrating inflammatory cells (36,37), thus rejecting ideas of being 'softened up'. That is presumably incorrect, since malignant cells when breaking out of the conformity of a regular epithelium do secrete enzymes, most crucially against collagen IV (38,39). This information, additionally, corroborates the specific mechanical contribution of this protein in maintaining structural integrity of basement membranes. Conversely, it is unlikely that transmigrating lymphocytes have such aggressive properties. Neither would the passive (?) movement of chylomicrons (0.5-1.0nm diameter) require such behaviour, despite their obvious movement through the membrane (40,41). A more likely explanation for these breaches probably rests in the physic-chemical make-up of the basement membrane itself which momentarily dissolves under local forces (rather like thixotropic "non-drip" paint which liquefies only under pressure from the paintbrush) (42).
Another intriguing relevant perspective, neither fully explored nor resolved, involves the presence of epithelial cell pseudopodia traversing the membrane to contact mesenchymal cells. It is probable that the marked increase in number and size of projections during end-gestational development (43) may well correlate with the activity of mesenchyme in promoting epithelial maturation and polarity, and hence functional capacity and potential (9,11,44). That momentary cross-talk between epithelium and mesenchyme, unfortunately, has neither been entirely corroborated nor defined.
To what extent, therefore, do messages from the mesenchyme control and direct epithelium? We have seen that a mesenchymally-derived basement membrane dynamically controls morphogenesis, cell differentiation and polarity, while also providing the structural basis for villi, crypts and the microvasculature of the lamina propria, so that tissue morphology is preserved in the absence of epithelium. Now, in terms of mucosal (coeliac) "flattening", the frequently asserted presumption that loss of epithelium explains altered surface topology, especially by immunologists (45,46) whose conclusion, that IEL primed for enterocyte cytolysis provides the answer, is hardly sufficient.
But as we have argued elsewhere (47,48) such views are highly suspect since mucosal re-organisation requires immense cooperation between all elements within the lamina, including marked revisions of the microvasculature and extensive alterations to all basement membranes offering support to endodermal and mesenchymal components. Hence, we need to be very sure, even in these immune-driven situations, whether the force for change comes totally, or partially, from controlling influences elsewhere, or more specifically perhaps, from within the mesenchyme itself. Indeed, the mesenchyme may be the originating force which controls tissue shape and integrity to a far greater extent than has ever been realised hitherto, even to contributing to the innate immune response of tissues: a point recently articulated by others (49,50,51).
The lamina propria & the myofibroblast subepithelial cell system
In stark contrast to other studies on mucosal morphology and the villous epithelium, detailed analyses of the lamina propria have not been so prominent, either in regard to normal functioning or to pathological processes. Although the lamina propria contains a variety of haemopoietically-derived infiltrating cells (eosinophils, basophils, neutrophils, lymphocytes), we know little about its basic content of residual structural cells, their associated macromolecular repertoire (matrix proteoglycans), and how these are altered by specific disease processes. Histologically, the lamina is difficult to analyse, rather more being regarded simply as the region lying between the crypts and forming the internal villous core.
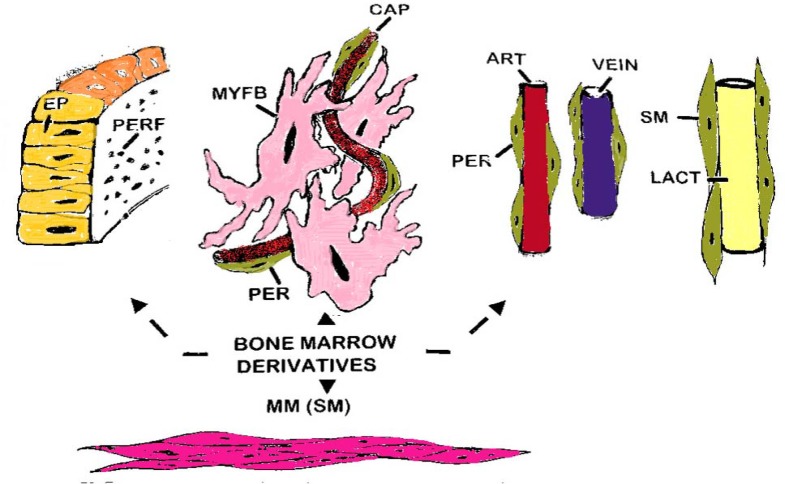
However, the lamina is bounded by a system of myofibroblasts which is intimately applied to the under-surface of the basement membrane, and directly connected with the subepithelial capillary vascular network of pericytes (52,53,54,55,56). In addition to various fibrous elements (including collagen IV, tenascin, desmin, entactin, laminins), the "ground substance" comprises a gel-like mixture of glycoaminoglycans of which hyaluronic acid is a prominent component. The degree of hydration of this matrix is dependent on the balance of absorbed fluid, secretions, and rate of removal through the microvasculature and lymphatics. Each villus is supplied by a central arteriole which branches into capillaries at its tip into a local tuft. This begins draining into a venule which takes origin from about the upper one-third of the villus. Below this tuft, the lower capillaries drain vertically downwards, subjacent to the basement membrane and fibroblast sheath, where they join vessels associated with the crypt mouths (57, 58): the dual structure of the subepithelial capillary sheath in human intestine should be noted. The central lacteal is clothed in smooth muscle cells in its upper part before its division into smaller tributaries, the latter only being supported by pericytes (also now considered to be part of the myofibroblastic system) (Figure 2).
Figure 2.
This diagram represents the types of bone-marrow cell derivatives operative within the lamina propria. They include (in cerise) the subepithelial myofibroblast system (MYF); pericytes (green) supporting the subepithelial capillaries and main vasculature of the villi (artery, red: vein, blue); the lacteal (L) supported by smooth muscle cells (SM) and (purple) the muscularis mucosae (MM). The basement membrane (green) is perforated (but artefactually so during processing for microscopy), comprising glycoglucosamines and fibres (such as collagen IV, tenascin, elastin, etc) which are all largely derivative of the mesenchymal cell populations illustrated
In view of recent important advances over the last few decades, attention should now be focussed on the myofibroblast system throughout the lamina propria and the different phenotypes emerging from within that system (59,60,61). It comprises a ubiquitous population of cells (62) that is usually α-SMActin + (smooth muscle), tenascin-C+ and desmin+, but in intense inflammation, as with Crohn's or IBD disease (63), they are prone to lose these markers when de-differentiating into fibroblasts which may also therefore be the source of intestinal fibrosis and stricture formation.
Their precursors could all derive from the bone marrow (64). This was demonstrated in an ingenious use of intestinal biopsy material from female patients thought to be developing GVHD after receipt of whole blood transfusions from male donors. Using the Y chromosome as evidence, they detected α-SMA+ donor cells within the pericryptal myofibroblast sheath of three biopsies. Therefore, these migrating cells probably arose from stromal cells, and possibly from among circulating "fibrocytes" (65) within the bloodstream. Interestingly, although not formally interrogated, these donor cells appeared to reach onto the villi, so the possibility of their migratory potential has not yet been entirely ruled out.
Subepithelial myofibroblasts, histologically, reveal a smooth muscle appearance but which variously (according to organ/tissue type) fulfil important regulatory activity in terms of tissue morphogenesis; remodelling (following injury); control of epithelial cell development, polarity and functional attributes; and an intimate involvement in repair, inflammation and fibrosis. In addition to the subepithelial fibroblast network, this group of cells also provides the pericytes in support of the microcirculation, as well as the vertically-aligned smooth muscle supporting the lacteal system within each villus. Their contractile properties are thus probably responsible for the contraction of individual villi, as presumably indicated by their 'fir-tree' profiles seen histologically, and in the horizontally-disposed folds revealed by scanning EM, thereby encouraging effective fluid transfer, and probably also aiding loss of cells from the epithelial surfaces.
Evaluating the villus – from normal to abnormal
This brief review emphasises the complexity of the structural, cellular, non-cellular, and gene-based aspects of villus-orientated biology, exemplifying, over the last 50 years, the vast expansion in knowledge applicable to its various parts. In addition, the villus is subject to varied influences, as typified by responses to gluten ingestion in genetically-predisposed individuals (66,67); high bacterial intestinal loads resulting in the host-directed syndrome of tropical ("sprue") enteropathies (68,69); or parasites - especially Giardia species (70), all of which evoke profound changes in villous morphology. While some of these changes are minimal, others result in significant re-modelling of the mucosa architecture: the spectrum of immunopathologic changes is tabulated for comparison (Table 1).
Table 1.
Some prominent pathologies leading to villus deformities
| «Normal»/ Preinfiltrative Marsh 0 |
Infiltrative Marsh I |
Infiltrative/ Hyperplastic Marsh II |
«Flat»/ Mosaic Marsh III |
«Flat»/ Unresponsive Marsh IV |
|
|---|---|---|---|---|---|
| Disorder | |||||
| Gluten hypersensitivitya | + | + | + | + | + |
| Tropical sprueb | +/- | + | + | + | - |
| Chronic diarrhoea/Marasmusc | +/- | + | + | + | - |
| Giardiasis/infectionsd | + | + | + | + | - |
| GVHDe | - | + | + | + | - |
Food antigensf
|
- - - - |
+ - + - |
+ - + - |
+ - + + |
- - - - |
Transport and enzyme disordersg
|
+ + + |
- - - |
- - - |
- - - |
- - - |
Immunodeficienciesh
|
+ | + | +/- | - | - |
| IBDi | + | + | + | - | - |
| Drugs (NSAIDs)j | + | + | + | +/- | - |
Neonatal enteropathiesk
|
+ + + |
+/- - - |
+/- - - |
+/- - - |
- - - |
(i) These gluten-induced and other hypersensitivity reactions lead to alterations in villous shape, ultimately being involved in the hyperplastic remodelling of mucosa into mosaic plateaux. Villi do not undergo atrophy, however, and neither is the mucosa subject to any atrophic process, since structural recovery ensues following use of a gluten-free diet:
(ii) The minimal change mucosal lesions (Marsh I and II) cannot be non-specific, because they represent specific host responses to identified inciting antigens:
(iii) The subdivision of Marsh III (a, b, c) has been shown in various independent studies to have no practical value: this misinterpretation results from the failure to recognise the elevated mosaic plateaux which amalgamate villi into these lozenge-shaped blocks of tissue. It follows [see Note (i]) that attempted sub-classification of the severe Marsh III lesion are futile, and would be better to be abandoned:
(iv) Immunodeficiency’s, Inflammatory bowel diseases (IBD), particularly Crohn’s disease and drugs may cause intraepithelial lymphocytosis similar to the Marsh I and II lesions while flat mucosa is only rarely encountered.
Much of the work done on these conditions, although principally from a clinico-pathological, diagnostic viewpoint, has centred specifically on the epithelium and its lymphocytic infiltrations; the shape of the villi; on the nature of its severest changes ("flattening"); with very scant attention being given to the lamina propria. These progressive changes (related in the main to gluten-induced hyper-sensitivity reactions) and the relevant computer-aided morphometric data have recently been schematically illustrated (48) thereby indicating both the time-frame, and key locations within the mucous membrane at which these changes are progressively initiated.
Myofibroblasts, as we have shown, arise in the mucosa from circulating fibroblasts, and which in the presence of pro-inflammatory proteins synthesise extra-cellular matrix (ECM). If the latter is disorganised through tissue remodelling (as signally occurs in the evolution of the characteristic coeliac mucosa), fibroblasts acquire stress fibres, gradually being transformed into mature myofibroblasts expressing α-SMA. Myofibroblast differentiation requires three stimuli: a) TGF-β1); special ECM proteins like ED-A (a splice variant of fibronectin); and c) stress resulting from tissue remodelling (71,72). Despite that, and in contrast to pulmonary fibrosis, liver cirrhosis, or the chronic cicatrizing fibrosis of Crohn's disease, extensive fibrosis is not a major feature of celiac mucosae, the former possibly due to a breakdown in epithelial-to-mesenchymal (EMT) transitions (73).
EMT interactions are essential in foetal intestinal development and later adult architecture, a process critically dependent on epithelial Hedgehog (Hh) in the formation of the lamina propria. Hh binds to Patched (Ptch) on target cell membranes (74, 75). Indeed in studies of mice programmed for chronically-reduced Hh signalling, the result was the development of diarrhoea, malabsorption, weight loss and malnutrition accompanied structurally by a reduced villous heights, crypt hypertrophy and inflammation in the lamina (76). In addition, there was loss of smooth muscle leading to failure of lacteal development, here analogous with intestinal lymphangiectasia.
The purpose of this essay was not primarily to notice the acquired pathology of diseases affecting the villus. Nonetheless, these very brief remarks illustrate just how little is known about the remodelling of the mucosa in terms of genetic activation or deficiency, indicating the vast chasm which needs to be investigated, understood and further integrated into current understandings of mucosal disease. Their relevance to celiac disease is immediately apparent, and again with observations which distract strongly away from the idea that mere "atrophy" has anything to do with these profound tissue re-arrangements.
However, in light of this review, it is clear that much more work needs to be done on small bowel enteropathies regarding changes in the lamina propria and the role taken by the syncytial subepithelial myofibroblast sheath. Mucosal re-modelling is not simply related to loss of epithelial cells, as often widely assumed. This, therefore, remains a challenge. It requires further investigation of the Hedgehog and Wnt series of genes (77) which exert such major influences on mucosal structure, development, maintenance, and pathology. It is inconceivable that this feature of the mucosa does not exert a prominent role in the immunopathogenic changes which have already been described: some encouraging starts have already been made (78) and which should stimulate further research in this area, thus bringing newer insights into how these changes are brought about – and, of course, reversed following treatments.
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgment
The authors would like to express their appreciation for the elegant artwork performed by Ms. Defne Savaş in figures 1 & 2.
References
- 1.Cruveilhier J, See M, editors. Traite d'Anatomie. 5th Ed. Paris: Asselin; 1874. [Google Scholar]
- 2.Bichat X, editor. Traite des Membranes en General et de Diverses Membranes en Particuleur. Paris: ReInk Books; 1800. [Google Scholar]
- 3.Henle FGJ. Symbolae ad Anatomian Villorum Intestinalium Imprimis Eorum et Vasorum Lacteorum. Berolini; 1837. [Google Scholar]
- 4.Bowman W. "Mucous Membrane". In: Todd, RB, editor. Cyclopaedia of Anatomy & Physiology. Vol 3. London: Sherwood, Gilbert & Piper; 1847. [Google Scholar]
- 5.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main intestinal types in the mouse small intestine. Amer J Anat. 1974;141:461–562. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 6.Bertalanffy F, Nagy K. Mitotic activity and renewal rate of the epithelial cells of human duodenum. Acta Anat. 1961;45:362–370. doi: 10.1159/000141762. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald W, Trier J, Everett N. Cell proliferation in the stomach, duodenum and rectum of man: radioautographic studies. Gastroenterology. 1964;46:405–417. [PubMed] [Google Scholar]
- 8.Hermiston M, Green R, Gordon J. Chimeric-transgenic mice represent a powerful tool for studying how the proliferation and differentiation programs of intestinal epithelial cell lineages are regulated. Proc Nat Acad Sci [USA] 1993;90:8866–8870. doi: 10.1073/pnas.90.19.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbleib J, Sääf A, Brown P, Nelson W. Transcriptional modulation of genes encoding structural characteristics of differentiating enterocytes during development of a polarised epithelium in vitro. Mol Biol Cell. 2007;18:4261–4278. doi: 10.1091/mbc.E07-04-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang J, Chance M, Nicholas C, Ahmed N, Guileau S, Flandez M, et al. Protemic changes during cell maturation in vivo. J Protemics. 2008;71:530–546. doi: 10.1016/j.jprot.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellander H, Fändriks L. Surface area of the digestive tract – revisited. Scand J Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 12.Crawley S, Mooseker M, Tyska M. Shaping the intestinal brush border. J Cell Biol. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes R, Lobley R. Intestinal brush border revisited. Gut. 1989;30:1667–1678. doi: 10.1136/gut.30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh MN, Ensari A. The gut-associated lymphoid tissue and immune system. In: Whitehead R, editor. Gastrointestinal and Oesophageal Pathology. 2nd ed. Churchill Livingstone; 1995. pp. 201–225. [Google Scholar]
- 15.Holmes R. The intestinal brush border. Gut. 1971;12:668–677. doi: 10.1136/gut.12.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coch R, Leube R. Intermediate filaments and polarization in the intestinal epithelium. Cells. 2016;32:1–18. doi: 10.3390/cells5030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mooseker M. Organisation, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Ann Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 18.Noonan D, Fullet A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell J. The complete sequence of perlecan, a basement membrane heparin sulphate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–47. [PubMed] [Google Scholar]
- 19.Simon-Assmann P, Bouziges F, Arnold C, Haffen K, Kedinger M. Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development. 1988;102:339–347. doi: 10.1242/dev.102.2.339. [DOI] [PubMed] [Google Scholar]
- 20.Furuyama A, Mochitate K. Assembly of the exogenous extracellular matrix during basement membrane formation in vitro. J Cell Science. 2000;1113:859–868. doi: 10.1242/jcs.113.5.859. [DOI] [PubMed] [Google Scholar]
- 21.Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 22.Teller I, Auclair J, Herring E, Gautier R, Ménard , Beaulieui J-F. Laminins in the developing and adult human small intestine: relation with the functional absorptive unit. Develop Dynam. 2007;236:1980–1990. doi: 10.1002/dvdy.21186. [DOI] [PubMed] [Google Scholar]
- 23.Teller I, Beaulieu J-F. Interactions between laminin and epithelial cells in intestinal health and disease. Exp Rev Molec Med. 2001;3:1–18. doi: 10.1017/S1462399401003623. [DOI] [PubMed] [Google Scholar]
- 24.Schittny J, Timpl R, Engel J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparin sulphate proteoglycans in epithelial basement membranes of mouse cornea reveals different topological orientations. J Cell Biol. 1988;107:1599–1610. doi: 10.1083/jcb.107.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heremans A, van der Scheuren B, de Cock B, Paulsson M, Cassiman J-J, van der Berghe H, David G. Matrix-associated heparin sulphate proteoglycan: core protein-specific monoclonal antibodies decorate the pericellular matrix of connective tissue cells and the stromal side of basement membranes. J Cell Biol. 1989;109:3199–3211. doi: 10.1083/jcb.109.6.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn U. Facts and problems of the intestinal basement membrane. Digestion. 1990;46 (Suppl. 2):40–48. doi: 10.1159/000200365. [DOI] [PubMed] [Google Scholar]
- 27.Partridge B, Simpson L. Duodenal epithelial cell migration and loss in NZB mice. Micron. 1980;11:63–72. [Google Scholar]
- 28.Bullen T, Forrest S, Campbell F, Dodson A, Hershman M, Pritchard D, Turner J, Montrose M, Watson A. Characterisation of epithelial cell shedding from the human small intestine. Lab Invest . 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 29.Hall P, Coates P, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Science. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 30.Williams J, Duckworth C, Burkitt M, Watson A, Campbell B, Pritchard D. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Veterinary Pathol. 2015;52:445–455. doi: 10.1177/0300985814559404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker F, Barnes E, Kaye G. The pericryptal fibroblast sheath: IV – replication, migration, and differentiation of the subepithelial fibroblasts of the crypt and villus of the rabbit jejunum. Gastroenterology. 1974;67:607–621. [PubMed] [Google Scholar]
- 32.Marsh MN, Trier JS. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum: II – Radioautographic studies. Gastroenterology. 1974;67:636–645. [PubMed] [Google Scholar]
- 33.Neal J, Potten C. Description and basic cell kinetics of the murine pericryptal fibroblast sheath. Gut. 1981;22:19–24. doi: 10.1136/gut.22.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh MN. The scanning electron microscope and its application to the investigation of intestinal structure. In: Badenoch J, Brooke B, editors. Recent Advances in Gastroenterology. 2nd ed. Edinburgh: Churchill-Livingstone; 1972. pp. 81–135. [Google Scholar]
- 35.Komuro T. Fenestrations of the basal lamina of intestinal villi of the rat: scanning and transmission electron microscopy. Cell Tissue Res. 1985;239:183–188. doi: 10.1007/BF00214918. [DOI] [PubMed] [Google Scholar]
- 36.Marchesi V, Florey H. Electron microscopic observations on the emigration of leucocytes. Quart J Exp Physiol. 1960;45:343–347. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- 37.Florey H, Grant L. Leucocyte migration through small blood vessels stimulated with ultra-violet light: an electron microscope study. J Path Bact. 1961;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- 38.Liotta L, Abe S, Robey P, Martin G. Preferential digestion of basement membrane collagen by an enzyme derived from a metastatic murine tumor. Proc Nat Acad Sci [USA] 1979;76:2268–2272. doi: 10.1073/pnas.76.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liotta L, Tryggvason K, Garbisa S, Haert I, Foltz C, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 40.Casley-Smith J. The identification of chylomicra and lipoproteins in tissue sections and their passage into jejunal lacteals. J Cell Biol. 1962;15:259–277. doi: 10.1083/jcb.15.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin C. Electron microscope studies of triglyceride absorption in man. Gastroenterology. 1966;50:65–77. [PubMed] [Google Scholar]
- 42.Menefee M, Mueller C. Some morphological considerations of transport in the glomerulus. In: Dalton A, Haguenau F, editors. Ultratructure of the Kidney. New York: Academic ; 1967. p. 73. [Google Scholar]
- 43.Mathan M, Hermos J, Trier J. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972;52:577–588. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kedinger M, Simon-Assmann P, Alexandre E, Haffen E. Importance of a fibroblastic support for in vitro differentiation of intestinal endodermal cells and for their response to glucocorticoids. Cell Diff. 1987;20:171–182. doi: 10.1016/0045-6039(87)90431-3. [DOI] [PubMed] [Google Scholar]
- 45.Hüe S, Mention J-J, Monteiro R. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Setty M, Discepolo V, Abadie V, et al. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology. 2015;149:681–691. doi: 10.1053/j.gastro.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh MN, Rostami K. What is a normal mucosa? Gastroenterology. 2016;151:784–788. doi: 10.1053/j.gastro.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Marsh MN, Heal C. Evolutionary developments in interpreting the gluten-induced mucosal celiac lesion: an Archimedian heuristic. Nutrients. 2017;9:1–20. [Google Scholar]
- 49.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol (Gastro Liver Physiol) 1997;273:G769–G775. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 51.Otte J-M, Rosenberg I, Podolsky D. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 52.Kaye G, Lane N, Pascal R. The colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue II: Fine structural aspects of normal rabbit and human colon. Gastroenterology. 1968;54:852–865. [PubMed] [Google Scholar]
- 53.Marsh MN, Trier J. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum I Structural features. Gastroenterology. 1974;67:622–635. [PubMed] [Google Scholar]
- 54.Desaki J, Fujiwara T, Komuro T. A cellular reticulum of fibroblast-like cells in the rat intestine: scanning and transmission electron microscopy. Arch Histol Japonica. 1984;47:179–186. doi: 10.1679/aohc.47.179. [DOI] [PubMed] [Google Scholar]
- 55.Joyce N, Haire M, Palade G. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987;92:68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- 56.Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa) Arch Histol Cytol. 1990;53:1–21. doi: 10.1679/aohc.53.1. [DOI] [PubMed] [Google Scholar]
- 57.Casley-Smith J, Gannon B. Intestinal microcirculation: spatial organization and fine structure. In: Granger N, Shepherd A, editors. Physiology of the Intestinal Circulation. Raven Press; pp. 9–31. [Google Scholar]
- 58.Gannon B, Rogers P, O'Brien P. Two capillary plexuses in human intestinal villi. Micron. 1980;11:447–448. [Google Scholar]
- 59.Schmitt-Gräff A, Desmoulière A. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic plasticity. Virch Arch. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- 60.Powell D, Pinchuk I, Saada J, Chen X, Mifflin R. Mesenchymal cells of the lamina propria. Ann Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinz B, Phan S, Thannickal V, Galli A, Bochaton-Piallat M, Gabbiani G. The myoblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powell D, Mifflin R, Valentich J, Crowe S, Saada J, West J. Myofibroblasts I Paracrine cells important in health and disease. Am J Physiol (Cell Physiol) 1999;46:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 63.Francoeur C, Bouatrouss Y, Seltana A, Pinchuk I, Vachon P, Powell D, Sawan B, Seidman E, Beaulieu F-J. Degeneration of the pericryptal myofibroblast sheath by proinflammatory cytokines in inflammatory bowel disease. Gastroenterology. 2009;136:268–277. doi: 10.1053/j.gastro.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 64.Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes S, Hodivala-Dilke K, Goldman J, Alison M, Wright N. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe R, Donnelly S, Peng T, Bucala R, Metz C. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 66.Marsh MN. Grains of truth: Evolutionary changes in small intestinal mucosa in response to environmental antigenic challenge. Gut. 1990;36:111–114. doi: 10.1136/gut.31.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsh MN. Gluten, major histocompatibilty complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten-sensitivity ("celiac sprue") Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 68.Baker SJ. Idiopathic small intestinal disease in the tropics. In: Chandra RK, editor. Critical reviews in Tropical Medicine . Vol 1. New York: Plenum; 1982. pp. 197–245. [Google Scholar]
- 69.Marsh MN. Celiac and allied sprue syndromes [Absorption and Malabsorption] . In: Sleisenger MH, Fordtran JS, editors. Seminars in Gastrointestinal Disease. 1992. pp. 214–223. [Google Scholar]
- 70.Roberts-Thomson IC. Giardiasis: the role of immunological mechanisms to host-parasite relationships. In: Immunopathology of the Small Intestine, Marsh MN., editors. New York: Wiley; 1987. pp. 209–222. [Google Scholar]
- 71.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–181. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Tomasek J, Gabbiani G, Hinz B, Chaponnier C, Brown R. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 73.Powell D, Pinchuk I, Saada J, Chen X, Mifflin R. Mesenchymal cells of the intestinal lamina propria. Ann Rev Physiol. 2011;73:2131–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Brink G. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 75.van Dop W, Uhmann A, Wijgarde M, Sleddens-Linkels E, Heijmans J. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology. 2009;136:2195–2203. doi: 10.1053/j.gastro.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 76.Zacharias W, Li X, Madison B, Kretovich K, Kao J. Hedgehog is an anti-inflammatory epithelial signal for intestinal lamina propria. Gastroenterology. 2010;138:2368–2377. doi: 10.1053/j.gastro.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Madison B, Zacharias W, Kolterud Ä, States D, Gumucio D. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 78.Senger S, Sapone A, Fiorentino M, Mazzarella G, Lauwers , Fasano A. Celiac disease histopathology recapitulates hedgehog down-regulation, consistent with wound healing processes activation. Plos One. 2015;10:1–15. doi: 10.1371/journal.pone.0144634. [DOI] [PMC free article] [PubMed] [Google Scholar]