Figure 4.
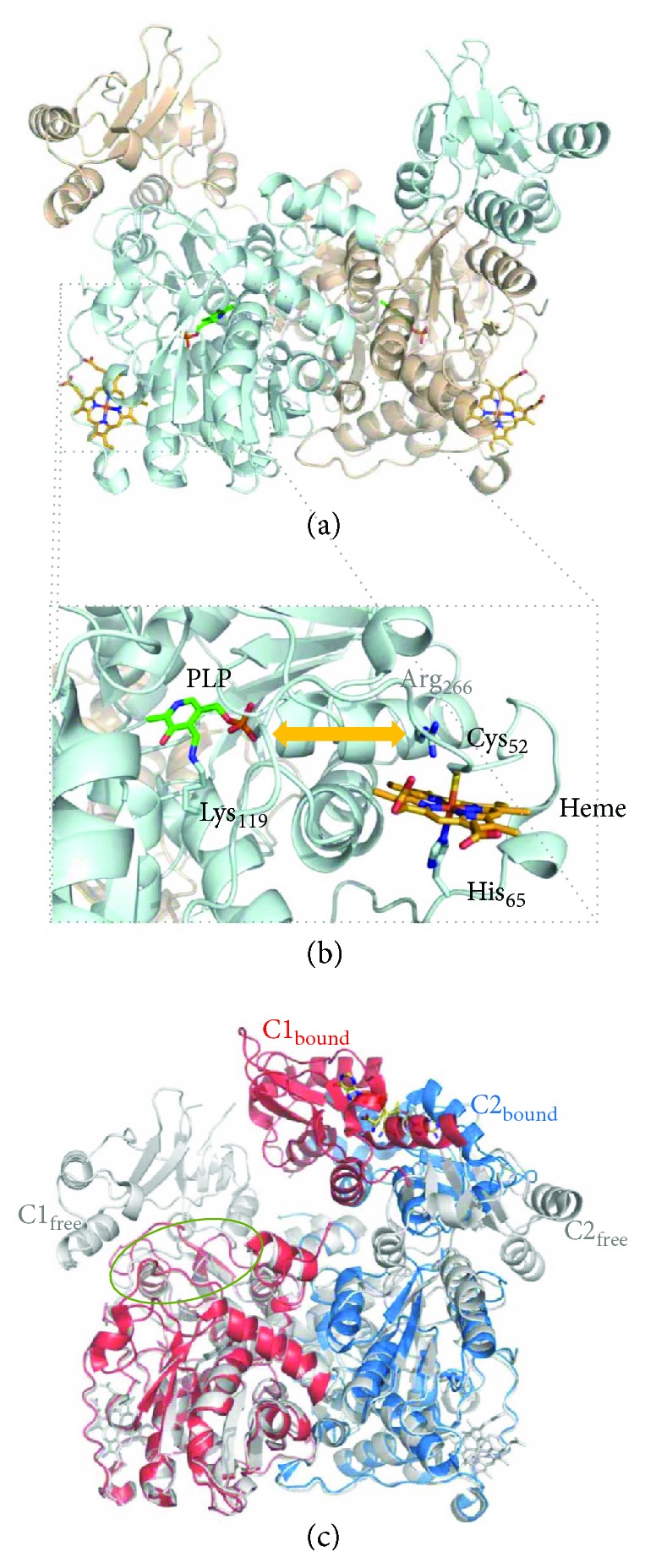
Crystallographic structure of human cystathionine β-synthase (CBS). (a) Cartoon representation of the “full-length” human CBS homodimer (PDB ID: 4COO; Δ516–525; 2.0 Å resolution). Green sticks, active site PLP moiety, where H2S production occurs. Orange sticks, regulatory heme b where CO or NO binds, resulting in enzyme inhibition. (b) Zoom-in into the catalytic (PLP) and regulatory (heme) sites. The PLP moiety is covalently attached to CBS through Lys119 (human CBS numbering), while the b-type heme is axially coordinated by Cys52 and His65. Orange arrow indicates the communication between the heme and PLP sites mediated by the α-helix comprising residues Thr257-Gly258-Gly259-Thr260-Ile261-Thr262-Gly263-Ile264-Ala265-Arg266. (c) Structural effect of AdoMet binding. While in AdoMet-free CBS, the C-terminal domain of each monomer (C1free and C2free, colored in light gray) blocks the substrate entrance into the active site (green oval circle) of the adjacent monomer, AdoMet binding to the C-terminal domains leads to association of the latter in a disk-like form (C1bound and C2bound; PDB ID: 4PCU; Δ516–525 Glu201Ser; 3.58 Å resolution), unblocking the active site and derepressing the enzymatic activity. Figure generated with PyMol 1.8.2.0 [238].
