Figure 8.
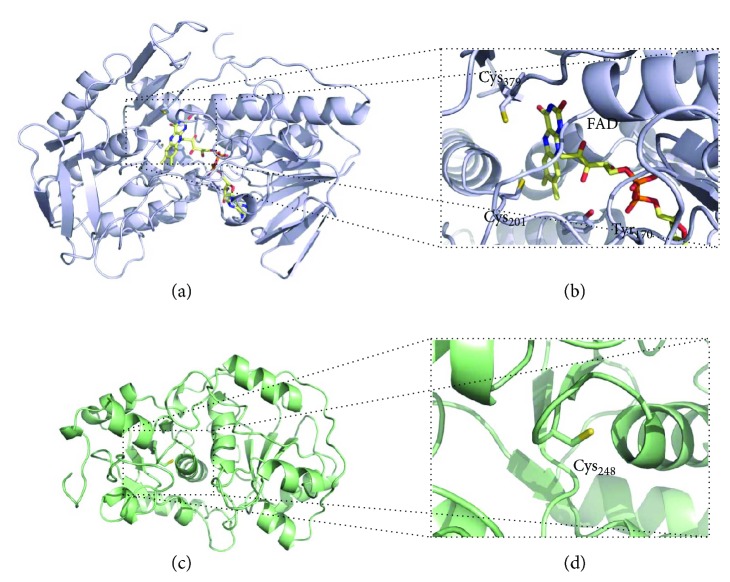
Structural models of human sulfide quinone oxidoreductase (SQR) and rhodanese (Rhod). (a) Cartoon representation of structural model of human SQR (UniProt accession code: Q9Y6N5.1) generated with Swiss-Model based on the structure of Acidithiobacillus ferrooxidans SQR (H198A variant; PDB code: 3SZF; ~19% sequence identity; ~87% sequence coverage) [78]. Flavin adenine dinucleotide (FAD) cofactor in yellow sticks. (b) Zoom-in on the SQR active site comprising the FAD moiety, the active site cysteine residues Cys201 and Cys379, and the Tyr170 residue which may establish a covalent link with the FAD cofactor. (c) Cartoon representation of structural model of human rhodanese (UniProt accession code: Q16762.4) generated with Swiss-Model based on the structure of the bovine enzyme (PDB code: 1BOH; ~90% sequence identity; 100% sequence coverage). (d) Zoom-in on the Rhod Cys248 active site. Figure generated with PyMol 1.8.2.0 [238].