Abstract
Cortisol is a well-known glucocorticoid that can be used as a biomarker of hypothalamic-pituitary-adrenocortical activity. To explore basal cortisol physiology during pregnancy and infancy in Macaca nemestrina monkeys, hair was collected from a convenience sample of 22 healthy mother-infant dyads. Adult females were housed in pairs as part of a small breeding colony at the Washington National Primate Research Center and infants were reared in a specialized nursery. Maternal samples were collected from females during a pregnancy-detection ultrasound and immediately following labor and delivery. Infant samples were collected at birth, 20 days, 4, 6, 8 and 10 mos. of age. Hair cortisol concentrations (HCCs) were determined using an enzyme immunoassay in washed and ground hair samples. Like human mothers, macaque HCCs rose during pregnancy (paired t=5.8, df=16, p<.001). Maternal HCCs at pregnancy-detection (114.2 ± 12.07 picogram/milligram (pg/mg)) were highly predictive of maternal HCCs at delivery (144.8 ± 13.60 pg/mg), suggesting a trait-like quality (r=0.90, P<.001). When maternal HCCs were viewed on a continuum, the absolute rise in cortisol over the course of pregnancy was significantly related to newborn HCCs (r=0.55, P=.02). Infant birth HCCs (1027.4.3 ± 97.95 pg/mg) were seven times higher than maternal HCCs at delivery (paired t = 19.1, df = 16, P<0.001). Higher birth HCCs were strongly associated with larger decreases in infant hair cortisol until 6 months of postnatal age when infant HCCs converged on values indistinguishable from adults. Overall, study results demonstrate a marked degree of fetal cortisol exposure during the latter part of gestation and suggest that the rise in maternal cortisol over pregnancy may play an influential role on HCCs in the newborn.
Keywords: cortisol, hair, pregnancy, fetal exposure, infant, macaque
Introduction
Glucocorticoids represent a class of steroid hormones that have an array of biological effects and are essential for survival and adaptation [Myers et al., 2014]. Cortisol is the most abundant glucocorticoid and plays a number of vital roles in health that include immune system suppression, maintenance of blood sugar and the metabolism of fat, protein and carbohydrates. In diurnal species, cortisol follows a 24 hour circadian rhythm wherein levels are highest during the morning (approx. 30 min after awakening in humans) and progressively decrease over the day [Corbalan-Tutau et al., 2014]. Environmental stimuli that signal real or perceived threats lead to increased cortisol secretion by the hypothalamic-pituitary-adrenal (HPA) axis, and cortisol concentrations in a variety of biological mediums are used as a surrogate for the measurement of psychological stress and adversity in many species including humans [Weinstock, 2008; Wosu et al., 2013].
The use of hair for the quantitative measurement of cortisol has received increasing recognition in fields such as wildlife biology, ecology, toxicology, forensic science, epidemiology, psychology and medicine over the last several decades [Bévalot et al., 2000; Raul et al., 2004; Kirschbaum et al., 2009; Lafferty et al., 2015; Wester et al., 2015]. The biological mechanisms that allow cortisol to be incorporated into the growing hair shaft are not fully understood, but cortisol present in circulating blood can diffuse into the growing hair shaft and is likely a primary method of incorporation [Meyer & Novak, 2012]. Rather than reflecting cortisol output over hours or days, samples of terminal hair provide a means to measure cortisol activity over the last several months (hair grows an average of about 1cm/mo both in humans and in macaque monkeys) [Dolnick, 1969; Wennig, 2000].
The use of hair cortisol in nonhuman primates has intriguing possibilities for characterizing long-term psychological wellness and evaluating stress management in captive populations [Novak et al., 2013; Carlitz et al., 2014; Baker and Dettmer, 2016]. Like humans, stress in nonhuman primates has been shown to impact HPA activity and the production of cortisol. In rhesus macaques, concentrations of hair cortisol have been shown to be sensitive to stressors such as high population density [Dettmer et al., 2014]. Findings from this study suggest that increased population density is associated with elevated HCCs (hair cortisol concentrations) and that HCC is a viable biomarker of chronic stress in captive primate populations. This hormonal metric has the potential to be a particularly valuable tool for sensitive subgroups such as infants to provide retrospective information about hormone exposure during gestation. A seminal investigation led by Kapoor [Kapoor et al., 2014] demonstrated that hair can successfully be used to measure neuroendocrine function, including cortisol secretion, in pregnant rhesus females and their infants. In the macaque fetus, body hair begins growing around day 90–100 of gestation [Bell, 1969] so that newborn hair reflects cumulative exposure to maternal and fetal derived cortisol across the last two-to-three months of gestation. Neonatal and infant HCCs could potentially be used as a retrospective biomarker of the HPA response to challenges within the prenatal and postnatal environments, including in utero exposure to elevated levels of maternal cortisol [Kapoor et al., 2016, Meyer and Hamel, 2014]. Stalder and Kirschbaum [2012] have suggested that fetal exposure to increased concentrations of maternal cortisol should be considered within the category of relevant environmental exposures that can have enduring effects on health, development and susceptibility to disease.
In this manuscript, we detail a collaborative study between the University of Washington and the University of Massachusetts-Amherst that was designed to provide longitudinal survey data on the normative production of hair cortisol in captive Macaca nemestrina mother-infant dyads. Hair was selected to measure cortisol because it can be obtained noninvasively, is easily collected at all age points and is not affected by time of day or current environmental factors. Samples were obtained from a convenience sample of healthy Macaca nemestrina monkeys over pregnancy and their nursery-reared infants over the first ten months of postnatal life. Adult females were not subjected to an imposed stressor during pregnancy, so study results provide normative basal hair cortisol values at key physiological time points in this species. Based on the results of previous studies, we hypothesized that maternal HCCs would increase during pregnancy and that newborn HCCs would be higher than maternal levels at delivery. We also hypothesized that there would be a significant positive relationship between maternal and newborn HCCs, illustrating the influence of maternal hormone levels on cortisol deposition in the fetus.
To test our hypotheses, we collected samples to answer the following questions:
Question #1: How do maternal HCCs change during pregnancy?
Question #2. How do changes in maternal HCCs influence newborn HCCs?
Question #3. How do HCCs change during infancy (over the 1st 10 months of life)?
Question #4. How do newborn HCCs influence the change in HCCs during infancy?
Methods
Subjects:
We initiated an investigation of basal cortisol physiology during pregnancy and infancy in a convenience sample of pregnant Macaca nemestrina monkeys and their infants at the University of Washington’s National Primate Research Center. From a convenience sample of 22 separate mother-infant dyads, we were able to collect maternal hair samples at pregnancy detection and maternal and newborn hair samples at delivery from 17 mother-newborn dyads to address questions #1 and #2 (maternal–newborn analysis). To address questions #3 and #4, we were able to collect hair samples from 18 infants over the first 10 months of life (infancy analysis). Thirteen infants were included in both the maternal-newborn and the infancy analyses samples (see Table 1). Adult female subjects were pair-housed and regularly provided with fresh fruit, foraging material, treats and enrichment toys in their homecages and monitored by trained staff on a daily basis.
Table 1:
List of subject characteristics and hair cortisol concentrations for maternal-newborn (N=17) and infancy (N=18) analyses.
| Hair Cortisol Concentrations (pg/mg) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother-Infant Dyad | Primiparous or Multiparous |
BirthDate | Delivery Type | Experimental Intervention1 | Infant Gestation Age at Birth | Infant Sex | Birthweight | Maternal Pregnancy Detection | Maternal Delivery | Maternal Difference2 | Maternal % Difference3 | Infant Birth | 20 Days |
4 Months | 6 Months | 8 Months | 10 Months |
| Mother-Infant Dyads in Maternal-Newborn and Infancy Analyses | |||||||||||||||||
| 1 | Multiparous | 07/05/13 | C-section | No | 178 | Male | 660 | 229.0 | 238.3 | 9.3 | 4.1% | 601.4 | 319.4 | 194.6 | 119.4 | 89.4 | 81.3 |
| 2 | Primiparous | 07/17/13 | C-section | Yes | 173 | Female | 535 | 218.1 | 232.6 | 14.5 | 6.6% | 655.0 | 423.6 | 253.8 | 160.8 | 100.7 | 97.1 |
| 3 | Multiparous | 07/20/13 | C-section | No | 179 | Male | 495 | 52.2 | 76.6 | 24.4 | 46.7% | 771.5 | 446.0 | 74.1 | 86.8 | 67.3 | 52.8 |
| 4 | Multiparous | 07/22/13 | C-section | Yes | 180 | Female | 559 | 103.2 | 110.0 | 6.8 | 6.6% | 964.8 | 133.3 | 123.4 | 160.0 | 113.8 | 118.5 |
| 5 | Multiparous | 08/10/13 | Natural | No | 168 | Female | 480 | 143.3 | 245.1 | 101.8 | 71.0% | 1509.2 | 293.8 | 151.6 | 147.2 | 121.9 | 129.7 |
| 6 | Primiparous | 09/03/13 | C-section | No | 179 | Male | 600 | 106.3 | 198.0 | 91.7 | 86.3% | 1441.9 | 512.3 | 146.4 | 79.4 | 129.0 | 116.9 |
| 7 | Multiparous | 09/17/13 | Natural | No | 180 | Female | 500 | 56.9 | 81.5 | 24.6 | 43.2% | 727.4 | 149.5 | 185.6 | 127.4 | 106.2 | 94.7 |
| 8 | Primiparous | 10/02/13 | Natural | Yes | 174 | Male | 535 | 63.2 | 93.2 | 30.0 | 47.5% | 833.3 | 179.5 | 182.3 | 121.0 | 114.4 | 117.0 |
| 9 | Primiparous | 10/20/13 | Natural | No | 173 | Female | 485 | 101.8 | 136.5 | 34.7 | 34.1% | 1241.6 | 453.2 | 298.8 | 97.9 | 86.3 | 139.9 |
| 10 | Primiparous | 12/08/13 | Natural | Yes | 173 | Male | 480 | 107.1 | 131.1 | 24.0 | 22.4% | 1046.4 | 220.5 | 157.1 | 87.3 | 92.8 | 122.8 |
| 11 | Multiparous | 06/15/14 | Natural | No | 178 | Male | 640 | 102.6 | 126.3 | 23.7 | 23.1% | 890.0 | 227.5 | 149.7 | 165.7 | 119.2 | 105.7 |
| 12 | Primiparous | 08/01/14 | Natural | No | 180 | Female | 490 | 106.1 | 112.5 | 6.4 | 6.0% | 663.6 | 271.3 | 122.9 | 121.4 | 133.1 | 116.4 |
| 13 | Primiparous | 09/09/14 | Natural | Yes | 179 | Female | 545 | 110.1 | 150.1 | 40.0 | 36.3% | 1500.0 | 343.3 | 141.0 | 131.2 | 88.8 | 76.3 |
| Mother-Infant Dyads in Maternal-Newborn Analysis Alone | |||||||||||||||||
| 14 | Primiparous | 08/06/13 | Natural | No | 163 | Female | 380 | 92.6 | 129.2 | 36.6 | 39.5% | ||||||
| 15 | Multiparous | 01/04/15 | Natural | No | 176 | Female | 540 | 164.3 | 190.0 | 25.7 | 15.6% | ||||||
| 16 | Multiparous | 07/18/15 | C-section | Yes | 185 | Female | 520 | 108.7 | 132.4 | 23.7 | 21.8% | ||||||
| 17 | Primiparous | 07/14/15 | Natural | No | 175 | Female | 605 | 75.9 | 78.8 | 2.9 | 3.8% | ||||||
| Mother-Infant Dyads in Infancy Analysis Alone | |||||||||||||||||
| 14 | Multiparous | 06/01/13 | Natural | No | 171 | Female | 490 | 1335.4 | 378.0 | 163.8 | 192.1 | 148.9 | 138.9 | ||||
| 15 | Primiparous | 06/13/13 | C-section | No | 179 | Female | 470 | 817.6 | 324.5 | 186.1 | 122.7 | 130.9 | 120.5 | ||||
| 16 | Primiparous | 08/30/13 | C-section | No | 178 | Female | 500 | 686.8 | 250.7 | 215.7 | 171.5 | 133.7 | 124.0 | ||||
| 17 | Primiparous | 04/16/14 | Natural | No | 172 | Female | 595 | 934.0 | 233.2 | 103.9 | 118.6 | 103.6 | 118.6 | ||||
| 18 | Multiparous | 06/03/14 | Natural | No | 175 | Female | 515 | 1005.6 | 250.7 | 135.2 | 136.3 | 150.0 | 131.1 | ||||
Experimental Intervention = Females were tested for wearing a jacket for tethering study during pregnancy
Maternal Difference Hair Cortisol Concentration = Maternal Delivery Hair Cortisol Concentration - Maternal Pregnancy Detection Hair Cortisol Concentration
Maternal % Difference Hair Cortisol Concentration = Maternal Difference Hair Cortisol Concentration / Maternal Delivery Hair Cortisol Concentration
As a convenience sample, the background of the females varied in terms of reproductive history and the level of intervention by human handlers during pregnancy. Nine of the adult females were primiparous and 8 were multiparous. No animal experienced chronic stress during pregnancy but six females were temporarily assigned to a maternal-fetal immunologic study that required preconditioning to a nylon jacket. The length of the jacket preconditioning varied between animals but typically lasted from several days to two weeks before females were released back to the colony. All subjects were sedated for ultrasound pregnancy detection (approximately 30 days post-conception) and delivery as well as routine veterinary care procedures (e.g. TB testing). The 22 infants in our convenience sample (16 females, 6 males) were hand-raised in a specialized nursery that has developed husbandry procedures to successfully care for young primates [Sackett et al., 2006]. The gestational age (x̄=176 ± SE 1.02 days) and birthweight (x̄=528 ± SE 13.53 grams) of these infants were normal for the species and illustrate the healthy nature of the pregnancies within this cohort. The mean gestational age and birthweight of infants included in the maternal–newborn analysis and the infancy analysis were nearly identical (see Table 1). All procedures were conducted with the strictest adherence to ethical and regulatory standards set forth by the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Nonhuman Primates and were approved by the Institutional Animal Care and Use Committee at the University of Washington, Seattle, WA, USA.
Hair Collection:
To minimize the disturbance of sampling procedures on pregnant animals, hair samples were collected when the adult females were sedated for routine pre-and postpartum health procedures. This schedule allowed samples to be collected at pregnancy detection and at infant delivery. Hair samples from awake infants were obtained at birth, 20 days and 4, 6, 8 and 10 months.
Sampling Procedure:
Using clippers, the nape of the neck was gently shaved (3 cm by 3 cm) by trained laboratory personnel. The nape was defined as the bottom hair line at the back of the head. Repeated sampling from the same animal was always performed in the same location on the neck (i.e., shave-reshave procedure). Consequently, although the hair sample obtained from each dam at pregnancy detection indexed cortisol accumulation over an indefinite time period prior to shaving (since those samples contained hairs at varying stages in the growth cycle), the second sampling specifically captured cortisol accumulation during the period from pregnancy detection to parturition.
For the infants, the hair sample at birth indexed cortisol accumulation during the period from onset of fetal hair growth to parturition, whereas samples obtained at later time points captured cortisol accumulation during the inter-sampling interval. Samples were tightly wrapped in foil, clearly labeled and placed in a −80°C degree freezer until shipped for analysis. Using express mail, they were then shipped in non-refrigerated batches to the University of Massachusetts at Amherst for cortisol analysis. Cortisol is highly stable in hair and can be stored at room temperature for months, even years, without degradation [Yamanashi et al., 2016].
Cortisol Assay:
The procedures for the hair cortisol assay are detailed in published reports by Meyer et al., 2014 and Davenport et al., 2006. They have been successfully used in a number of published reports on cortisol values in feral and laboratory housed nonhuman primates [Novak et al., 2014; Dettmer et al., 2009, 2014, 2015]. Briefly, the samples were washed twice with isopropanol to remove external sources of contamination (for example, sweat or sebum) and air-dried. The cleaned hair samples were then powdered by using a ball mill (Retsch, Verder Scientific, Newtown, PA) to break up the hair’s protein matrix and increase the surface area for extraction. The entire length of the hair sample was used. Subsequently, approximately 50 mg of powdered hair from each sample was extracted overnight with methanol. Extracts were evaporated, reconstituted in assay buffer, and analyzed in duplicate for cortisol by enzyme immunoassay (Salimetrics, State College, PA). The intraassay coefficient of variation was 1.9% and the interassay coefficient of variation was 6.9%.
Data Analysis:
Data were analyzed using Systat Software (Systat Software, San Jose, CA). HCCs were positively skewed and were log transformed to improve normality of the distributions. The alpha level for reporting significance was p<0.05.
To address Question #1: How do maternal HCCs change during pregnancy, we performed a paired t-test comparing maternal HCCs at pregnancy detection and delivery. The relationship between values at these two time points was assessed by Pearson correlation. Independent t-tests between females subjected to jacket preconditioning and other females, and between primiparous and multiparous females were also conducted to assess any effect of these variables on HCCs at delivery.
To address question #2, how do changes in maternal HCCs influence newborn HCCs, we first performed paired t-tests comparing the newborn HCCs to the maternal HCCs at pregnancy detection and delivery for the 17 animals included in our maternal-newborn analysis. We then performed Pearson correlations to assess the relationships among newborn HCCs and maternal HCCs at pregnancy detection and delivery. We also examined whether the changes in maternal HCCs during pregnancy were related to newborn HCCs using Pearson correlations. We first examined the relationship between newborn HCCs and the percent maternal HCC increase during pregnancy. For this, we calculated the difference between the maternal HCCs at delivery and pregnancy detection. We then divided this difference by the HCC level at pregnancy detection to obtain the percentage increase score. We then examined the relationship between newborn HCCs and the absolute values of the increases in maternal HCCs during pregnancy. For this analysis, we calculated the difference between the maternal HCCs at delivery and pregnancy detection.
To address question #3, how do HCCs change during infancy, we performed a repeated measures ANOVA with infant HCC measures at birth, 20 days, 4 months, 6 months, 8 months and 10 months as repeated measures, and infant sex and maternal parity as between subjects factors to examine differences in HCCs over the first year of life. Post hoc tests for repeated measures were performed using Bonferroni corrections.
To address question #4, how do newborn HCCs influence the change in HCCs during infancy, we derived difference scores for changes in postnatal HCC over the first 10 months of life (birth cortisol minus cortisol at 20 days, 4, 6, 8 or 10 mos.). These absolute difference scores were divided by the infant’s birth HCC to obtain percentage change scores for each age. We then performed Pearson correlations to assess the relationships among newborn HCCs and changes in HCCs during infancy.
Results:
The results of the paired t-test comparing the maternal HCCs at pregnancy detection to HCCs at delivery indicated that there was a significant increase in maternal HCCs during pregnancy (paired t = 5.8, df = 16, P < 0.001). The mean maternal HCC at pregnancy detection was 114.8 ± SE 13.6 picogram/milligram (pg/mg), while the maternal HCC at delivery was 144.8 ± SE 13.60 pg/mg (see Figure 1).
Figure 1:
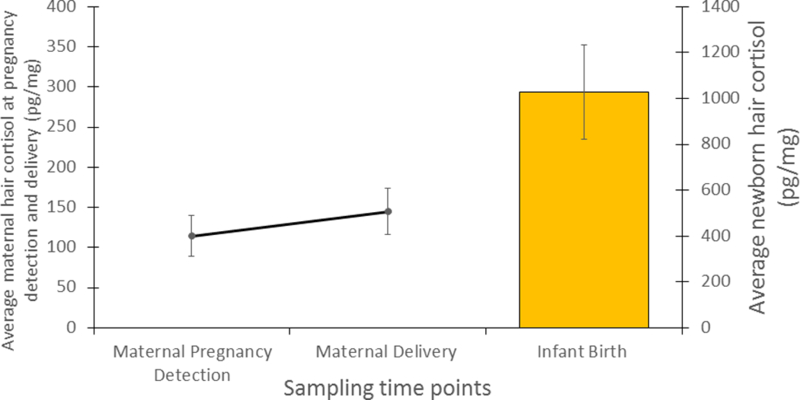
Average hair cortisol concentrations (pg/mg) for maternal and newborn babies (N=17), Errors bars are 95% confidence intervals.
Maternal HCCs at pregnancy detection and delivery were significantly correlated (r = 0.90, P< 0.001), suggesting strong intraindividual consistency within cortisol output patterns over pregnancy. Higher levels of HCCs at pregnancy detection were predictive of higher levels at delivery (see Figure 2). There were no differences between adult females with and without jacket preconditioning in maternal delivery samples (t = 0.02, df = 15, P = 0.98). Parity status (multiparous and primiparous) had no impact on maternal HCC values at pregnancy detection (t = 0.2, df = 15, P = 0.82) or delivery (t = 0.2, df = 15, P = 0.87) or on infant HCC values (t = −0.1, df = 15, P = 0.92).
Figure 2:
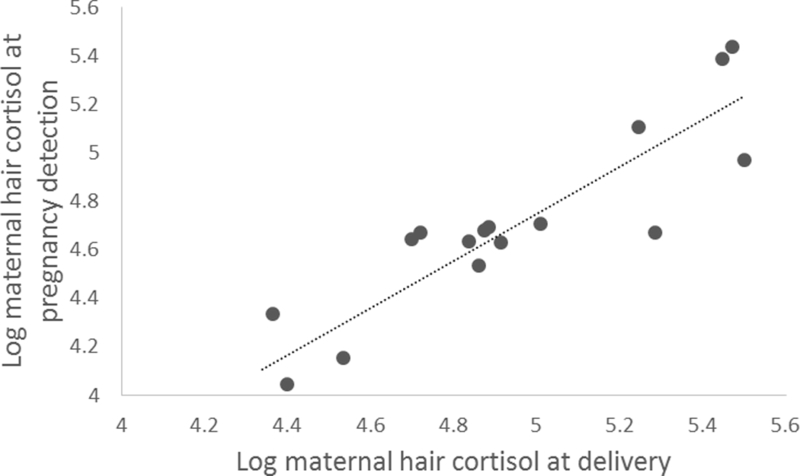
Scatterplot showing relationship between long maternal hair cortisol concentration at pregnancy detection and delivery (r=.90, p<.001).
The results of the paired t-test comparing newborn HCCs to maternal HCCs at pregnancy detection and delivery indicated that infant birth HCCs (1027.4 ± SE 97.05 pg/mg, N = 17) were significantly higher than maternal HCCs at pregnancy detection (paired t = 18.1, df = 16, P < 0.001) and delivery (paired t = 19.1, df = 16, P < 0.001) (see Figure 1).
The results of the Pearson correlations to assess the relationships among newborn HCCs and maternal HCCs at pregnancy detection and delivery indicated that newborn HCCs were not significantly correlated with maternal HCCs at pregnancy detection (r = .12, P = 0.63) or delivery (r = .33, P = 0.20). The percent increase in maternal HCCs during pregnancy was moderately, but not significantly, associated with newborn HCCs (r = 0.45, P = 0.07). The absolute values of increases in maternal HCCs however, were moderately and significantly correlated with newborn HCCs (r = 0. 55, P = 0.02) (see Figure 3).
Figure 3:
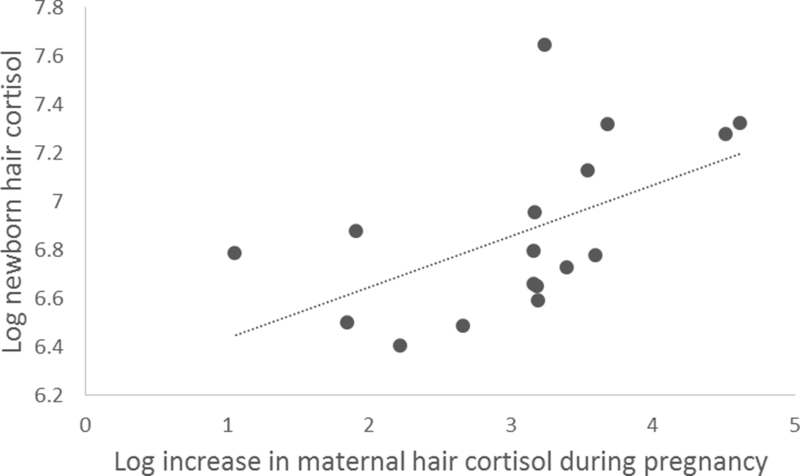
Scatterplot demonstrating the relationship between the rise in maternal hair cortisol concentrations over pregnancy and levels of newborn cortisol (r=0. 55, p=.02).
Infant HCCs dropped a precipitous 67% over the first twenty days of postnatal life and stabilized to reach values indistinguishable from adult females (at pregnancy detection) by approximately 6 months of age. By 6 months, infant HCCs averaged 130.4 ± SE 7.4 and represented a mere 15% of birth values. The results of repeated measures ANOVA indicated that the HCCs at birth and 20 days were higher than HCCs at all later time points. HCCs at month 4 were significantly higher than 8 month HCCs and 10 month HCCs (see Figure 4). There was no effect of maternal parity (primiparous vs multiparous; F = 0.3, df = 1,14, P = 0.61) or infant sex (F = 2.2, df = 1,14, P = 0.16) on infant HCCs at birth and throughout the first year of postnatal life.
Figure 4. Average (95% Cl) hair cortisol concentrations , Birth to 10 Months of Age.
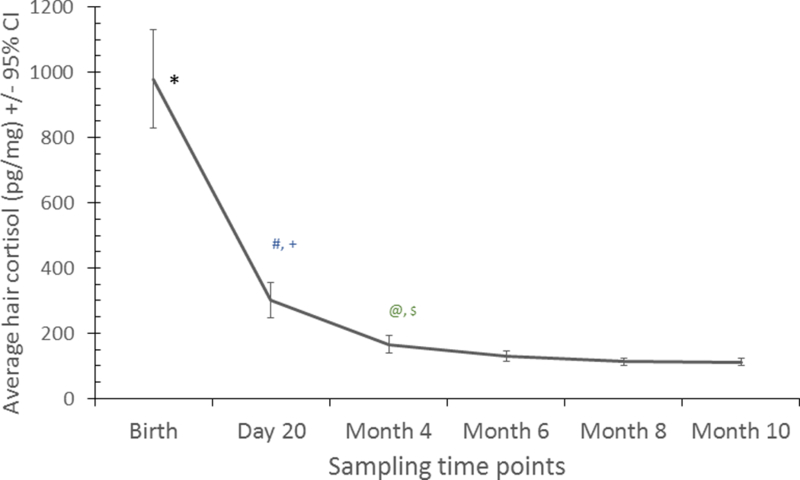
Results of tests: *Birth vs Day 20, Months 4,6,8,10 ( p<0.001 ), # Day 20 vs Month 4 (p<0.005), + Day 20 vs Months 6, 8, 10 (p<0.001), @ Month vs Month 8 ( p<0.05), $ Month 4 vs Month 10 (<0.005).
The results of Pearson correlations to assess relationships among newborn HCCs and percentage change in HCC during infancy indicated that newborn HCCs strongly influenced postnatal patterns of cortisol decline. Infant HCCs at birth were highly correlated with the decrease in infant HCCs over the first six months of life (see Figure 5). This finding suggests that higher birth HCCs were strongly related to larger decreases in HCCs sampled at 2, 4, 6, 8 and 10 months (see Table 2 for results of Pearson Correlations).
Figure 5:
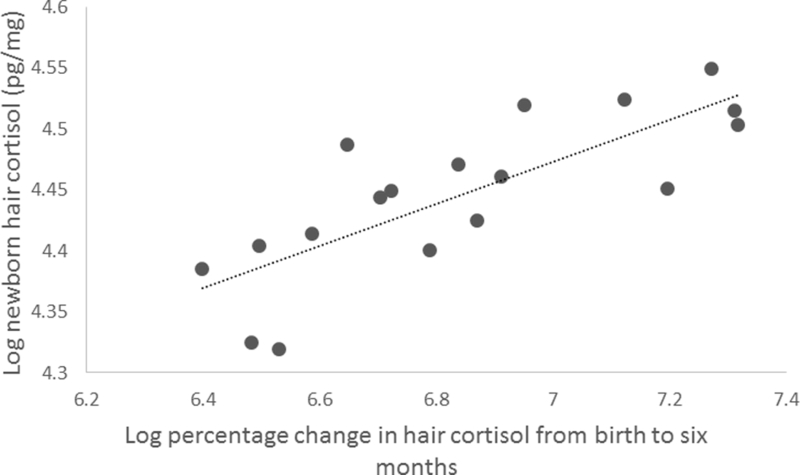
Scatterplot illustrating the relationship between newborn hair cortisol concentrations and change in hair cortisol from birth to 6 months of age (r=.79, p<.001).
Table 2:
Pearson (r) correlations for association between newborn hair cortisol (HC) concentrations (N=18) and decreases in hair cortisol during first 10 months of life
| 20 Day HC | 4 Month HC | 6 Month HC | 8 Month HC | 10 Month HC | |
|---|---|---|---|---|---|
|
Birth HC Pearson Correlation |
0.52* | 0.70**** | 0.79**** | 0.78**** | 0.73**** |
|
20 Day HC Pearson Correlation |
0.53* | 0.36 | 0.26 | 0.16 | |
|
4 Month HC Pearson Correlation |
0.74**** | 0.59** | 0.67*** | ||
|
6 Month HC Pearson Correlation |
0.83**** | 0.75*** | |||
|
8 Month HC Pearson Correlation |
0.90**** |
=p≤.05
=p≤.01
= p≤.005
=p≤.001
Discussion
In this study, we conducted a longitudinal survey of basal hair cortisol levels in a sample of healthy, Macaca nemestrina mother-infant dyads to provide normative values for the species and to explore the relationship between maternal HCCs and cortisol deposition in the infant. Results from this study provide support for our hypotheses on the pattern of cortisol deposition in maternal and fetal hair over pregnancy. Like human mothers, maternal HCCs in this species increase during pregnancy and there is strong intra-individual continuity in cortisol responses to pregnancy that suggest a trait-like quality in long-term HPA activity. Adult females with higher maternal HCCs at pregnancy detection continued to show higher HCCs at delivery. Maternal HCCs at pregnancy detection and delivery were not related to newborn HCCs, birthweight or gestational age. Our results did, however, demonstrate that the absolute rise in maternal cortisol over pregnancy was significantly associated with newborn hair cortisol values. This is the first report in the literature to report such a finding and suggests there may be a strong maternal influence on the deposition of cortisol in fetal hair during gestation.
Consistent with results from a report by Kapoor [Kapoor et al., 2014], newborn HCCs were about seven times higher than that of their mothers. Infant HCCs decreased by 67% over the first twenty days of postnatal life and reached stable values indistinguishable from adult females by 6 months of age. Because birth HCCs showed a wide range of variability, neonatal trajectories of cortisol disposition (i.e., synthesis, degradation and elimination) had to vary widely between subjects to allow convergence on adult HCCs by 6 months of age. This finding suggests that functional maturation of the adrenal cortex in primates may be a highly canalized process [Waddington, 1959; Flatt, 2005]. Our finding of adult-like HCCs by 6 months of age contrasts with results from investigations by Dettmer et al., 2014 and Laudenslager et al., 2012 that showed infant and juvenile macaques have higher HCCs than adults. These inter-study differences may be due to factors such as infant rearing conditions (e.g. nursery, mother and peer), facility differences in colony management and species under study. A combination of these factors may have influenced the results found in this longitudinal study with Macaca nemestrina nursery-reared infants and as such, findings may not necessarily apply to macaques reared differently or other species within the Macaca genus.
Much of what is known about the developmental trajectory of cortisol and the HPA axis in primates has been garnered from studies measuring plasma cortisol concentrations. Plasma cortisol concentrations in fetal rhesus monkeys during late gestation (i.e., measured at 130–160 days gestation with an average gestation period of 165 days) are 2- to 3-fold lower than the levels measured in the pregnant dams [Beamer et al., 1972; Kittinger, 1974; Serón-Ferré et al., 1978; Mitchell et al., 1981]. Ontogenic studies of basal HPA function have found stable or slight decreases in blood cortisol concentrations between 2 and 24 weeks of postnatal age [Champoux et al., 1989; Higley et al., 1992; Clarke and Schneider, 1993] with an adult-like pattern of cortisol in place by one year [Sanchez et al., 2005; Barrett et al., 2009]. The developmental trajectory observed in our study differs from what has been published with plasma and suggests cortisol availability is in place by about 6 months postnatal, significantly earlier than studies using plasma cortisol would suggest. The divergence in results between studies using hair and plasma may reflect the fact that cortisol concentrations vary in different biological mediums (as discussed below) and hair cortisol is not consistently correlated with cortisol obtained from plasma or saliva (Meyer and Novak, 2012).
The existing literature on the developmental biology of the primate adrenal cortex, where cortisol is produced and secreted, has shown that human and nonhuman primates (e.g. macaques, baboons) share strong similarities in the development of the fetal adrenal cortex [Ishimoto and Jaffe, 2011]. The fetal adrenal cortex has two distinct zones, the fetal zone (about 80–90% of the adrenals during gestation) and the definitive zone (widely considered to be the origin of adult adrenal cortices). During late gestation, the fetal zone is distinguished by rapid growth and the production of steroid hormones, including cortisol [Jaffe et al., 1981; Mesiano & Jaffe, 1997]. Enhanced formation of cortisol by the fetal adrenal gland is due to a developmental increase in the expression of 3β-hydroxysteroid dehydrogenase, an obligatory enzyme in the cortisol biosynthetic pathway [Sholl, 1983; Coulter et al., 1996]. Microscopic evaluations of adrenal cortices show a dramatic but orderly remodeling of morphology over the first postnatal year when the thickness of the fetal zone shrinks and the definitive zone grows in size. Adrenal weight dramatically increases during the last 2 weeks of gestation (concomitant with the late surge in fetal cortisol production), declines during the first 2 weeks postpartum due to shrinkage of the fetal zone and then remains relatively stable for a number of succeeding months as the cells within the definitive zone begin to reach structural and functional maturity [McNulty et al., 1981]. The pattern of postnatal infant HCCs described in this paper is consistent with the development of the macaque adrenal gland. Changes in infant adrenal maturation help account for the drop in infant HCCs from 20 days to 2 months, followed by only a slow change in HCCs at later ages tested.
The influence of maternal cortisol on neonatal cortisol concentrations has been somewhat controversial. Early work by Osinski [1960] and later studies by Slikker and colleagues [Slikker et al., 1982, Slikker et al., 1984] demonstrated that the placental barrier enzyme 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2), which converts cortisol to the inactive metabolite cortisone, acts to protect the fetus from exposure to high levels of maternal glucocorticoids. Despite the inactivation of maternal cortisol to cortisone by placental 11β-HSD2, radiotracer studies have shown that approximately 40–60% of fetal cortisol is of maternal origin, [Kittinger, 1974; Mitchell et al., 1981]. These findings suggest that much of the cortisol that can be measured in newborn macaque hair originates from the mother. As cortisol in hair is thought to reflect deposition from the free (unbound) fraction in the bloodstream [Meyer and Novak, 2012], the amount of cortisol binding to plasma proteins, particularly corticosteroid binding globulin (CBG), could also play a role in determining neonatal HCCs. Data from rhesus macaques indicates that plasma cortisol binding to CBG is lower at birth than in adulthood (i.e., a greater percentage of cortisol is unbound in the neonate) but rises over the first 1–2 years of life [Beamer et al., 1973]. Finally, although the infant’s own adrenal glands are presumably the primary source of cortisol deposited in hair from the day-20 time point onward, we note that in mother-reared infants cortisol can be transferred from the lactating dam to her offspring via milk, and that variation in milk cortisol levels has been linked to infant temperament [Sullivan et al., 2011; Hinde et al., 2015]. The amount of cortisol measured either in infant plasma or hair that derives from milk is not yet known.
One important goal of this study was to establish basal HCCs for macaque females and their infants, providing the detail that is necessary to describe the normative expression of hair cortisol over pregnancy and the first postnatal year. The results of this study are in concordance with data reported by Kapoor et al., [2014]. The range of maternal HCCs at delivery in the current study was 76.6 to 245.1 pg/mg with a mean of 144.8 ± 56.1 pg/mg (mean ± SD), while the average maternal HCC in the Kapoor cohort was 130 ± 46.53 pg/mg (mean ± SD) (no ranges provided). Pregnant females were housed in very similar laboratory environments in both studies. Newborns from the current investigation showed a range of 601.4 to 2082.5 pg/mg with a mean birth HCC of 1027.4 ± 400.1 pg/mg (mean ± SD) while the Kapoor cohort showed a mean newborn HCC of 1093 ±340 pg/mg (mean ± SD) (no ranges provided). These two normative biophysiology studies demonstrate that macaques have HCCs of about 1000 pg/mg at birth, values that are dramatically higher than those found in maternal hair samples collected at the same time point.
Several studies by Dettmer and colleagues [Dettmer et al., 2009, 2014, 2015] have examined hair cortisol in adult and infant rhesus macaques to investigate the relationship between HCCs and population density, maternal parity, other hair hormone concentrations and infant neurobehavioral development. A 2009 study by Dettmer et al reported an average HCC of 176.88 ± 13.08 pg/mg (mean ± SD) for six-month old, nursery-reared rhesus infants , a value that did not differ from mother and peer-reared animals of the same age (172.94 ± 12.36 mg/mg) [Dettmer et al., 2009]. Mean HCCs in the current study with 6 month old nursery-reared pigtail macaque infants averaged 137.3± 41.21 (mean ± SD), a value that also suggests nursery-reared animals do not display higher HCCs when compared to same-aged peers raised in more naturalistic rearing environments. While it is tempting to interpret this finding in the context of reduced psychosocial stress, Capitanio and co-authors have pointed out that there may be a generalized muting of the stress response in nursery-reared macaques that makes interpretation of cortisol values difficult [Capitanio et al., 2006]. A pattern of higher HCCs in primiparous dams during late pregnancy/early lactation found in a Dettmer 2015 investigation was not replicated in the current study. This divergence in results is likely due to differences in sample size, species or sampling time points. We also did not find an effect of maternal parity on infant HCCs. This may be the result of the standardized housing that our infants receive in the nursery, eliminating the influence of different care-taking styles that can characterize naïve and experienced macaque mothers.
The results of this longitudinal study provide substantial evidence that hair can be used as a hormone-bearing biological substrate to measure neuroendocrine (specifically adrenocortical) activity in Macaca nemestrina mother-infant dyads. The current findings demonstrate a marked degree of fetal cortisol exposure during the latter part of gestation and suggest that the rise in maternal cortisol over pregnancy may play an influential role on HCCs in the fetus. The pattern of hair cortisol decline during infancy suggests that an adult-like pattern of cortisol availability is in place by about 6 months of postnatal age, earlier than studies using blood cortisol would suggest. The different estimates of cortisol maturation gleaned from hair compared to blood sampling could be partially due to the above mentioned rise in CBG during postnatal development, a factor that would not directly influence plasma cortisol measurements but would presumably reduce the fraction of blood-borne cortisol available for incorporation into hair. The use of hair samples as a hormonal metric for animals and humans could be important for future studies of chronic stress, elevated cortisol output, and altered developmental programming in affected offspring. It also holds promise for exploring the long-term relationship between early hormone profiles and infant neurodevelopmental outcomes. Previous reports with rhesus macaques have reported that HCCs are predictive of early cognitive development [Dettmer et al., 2009} and higher maternal HCCs are associated with adverse changes in infant behavioral state (more irritable, less consolable) and lower sensorimotor reflex scores [Dettmer et al., 2015]. Our future study efforts will focus on increasing the sample size of our mother-infant cohort, expanding our investigation of HCCs in mother-reared infants and exploring the relationship between infant HCCs and measures of cognition, temperament and social development.
Acknowledgements:
We would like to express our sincere appreciation to Brenda Crouthamel, Noelle Liberato McKain, Grace Lee, Clayton Ferrier and Steve Ellis for their skilled assistance with the research reported in this manuscript.
This research was funded by NIH grants P51 OD010425 (David Anderson, PI), HD083091 (Mike Guralnick, PI) and R24OD011180 (Melinda Novak, PI).
References
- Baker KC, Dettmer AM. 2016. The well-being of laboratory non-human primates. American Journal of Primatology doi: 10.1002/ajp.22520. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. 2009. Early adverserearing experiences alter sleep-wake patterns and plasma cortisol levels injuvenile rhesus monkeys. Psychoneuroendocrinology 34(7):1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer N, Hagemenas FC, Kittinger GW. 1972. Protein binding of cortisol in the rhesus monkey (Macaca mulatta). Endocrinology 90(1):325–327. [DOI] [PubMed] [Google Scholar]
- Beamer N, Hagemenas FC, Kittinger GW. 1973. Development of cortisol binding in the rhesus monkey. Endocrinology 93(2):363–368. [DOI] [PubMed] [Google Scholar]
- Bell M 1969. The ultrastructure of differentiating hair follicles in fetal rhesus monkeys (Macaca mulatta) In: W Montagna, RL Dobson, editors. Advances in Biology of Skin, vol. IX, Hair Growth. Oxford: Pergamon Press; p 61–81. [Google Scholar]
- Bévalot F, Gaillard Y, Lhermitte MA, Pépin G. 2000. Analysis of corticosteroids in hair by liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography. B, Biomedical Sciences Applications 740(2):227–236. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Reberts JA. 2006. Nursery rearing and biobehavioral organization In “Nursery Rearing of Nonhuman Primates in the Twenty-first Century (eds, GP Sackett, GC Ruppenthaland K Elias) 191–213 New York, Springer Science. [Google Scholar]
- Carlitz EH, Kirschbaum C, Stalder T, van Schaik CP. 2014. Hair as a long-term retrospective cortisol calendar in orangutans (Pongo spp.): new perspectives for stress monitoring in captive management and conservation. General and Comparative Endocrinology 195:151–156. [DOI] [PubMed] [Google Scholar]
- Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. 1989. Hormonal effects of early rearing conditions in the infant rhesus monkey. American Journal of Primatology 19:111–117. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. 1993. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Developmental Psychobiology 26(5):293–304. [DOI] [PubMed] [Google Scholar]
- Corbalán-Tutau D, Madrid JA, Nicolás F, Garaulet M. 2014. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiology of Behavior 123:231–235. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Mason JI, Jaffe RB. 1996. Functional maturation of the primate fetal adrenal in vivo. II. Ontogeny of corticosteroid synthesis is dependent upon specific zonal expression of 3 beta-hydroxysteroid dehydrogenase/isomerase. Endocrinology 137(11):4953–4959. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology 147(3):255–61. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Novak MA, Meyer JS, Suomi SJ. 2009. Hair cortisol predicts object permanence performance in infant rhesus macaques (Macaca mulatta). Developmental Psychobiology 51(8):706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, Suomi SJ. 2014. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, Novak MA. 2015. Associations between Parity, Hair Hormone Profiles during Pregnancy and Lactation, and Infant Development in Rhesus Monkeys (Macaca mulatta). PLoS One. 10(7):e0131692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolnick EH. Variability of hair growth in Macaca mulatta 1969. In: W Montagna, RL Dobson, editors. Advances in Biology of Skin, vol. IX, Hair Growth. Oxford: Pergamon Press; p 121–128. [Google Scholar]
- Flatt T 2005. The evolutionary genetics of canalization. Quarterly Review of Biology 80(3):287–316. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. 1992. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry 32(2):127–45. [DOI] [PubMed] [Google Scholar]
- Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, Capitanio JP. 2015. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology 26(1):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Jaffe RB. 2011. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocrine Reviews 32(3):317–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe RB, Serón-Ferré M, Crickard K, Koritnik D, Mitchell BF, Huhtaniemi IT. 1981. Regulation and function of the primate fetal adrenal gland and gonad. Recent Progress in Hormone Research 37:41–103. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Lubach G, Hedman C, Ziegler TE, Coe CL. 2014. Hormones in infant rhesus monkeys’ (Macaca mulatta) hair at birth provide a window into the fetal environment. Pediatric Research 75(4):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Lubach GR, Ziegler TE, Coe CL. 2016. Hormone levels in neonatal hair reflect prior maternal stress exposure during pregnancy. Psychoneuroendocrinology 66:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. 2009. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34(1):32–37. [DOI] [PubMed] [Google Scholar]
- Kittinger GW. 1974. Feto-maternal production and transfer of cortisol in the rhesus (Macaca mulatta). Steroids 23(2):229–243. [DOI] [PubMed] [Google Scholar]
- Lafferty DJ, Laudenslager ML, Mowat G, Heard D, Belant JL. 2015. Sex, Diet, and the Social Environment: Factors Influencing Hair Cortisol Concentration in Free-Ranging Black Bears (Ursus americanus). PLoS One 10(11):e0141489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, Fairbanks LA. 2012. Developmental patterns of hair cortisol in male and female nonhuman primates: lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology 37(10):1736–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty WP, Novy MJ, Walsh SW. 1981. Fetal and postnatal development of the adrenal glands in Macaca mulatta. Biology of Reproduction 25(5):1079–89. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB. 1997. Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews 18(3):378–403. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Novak MA. 2012. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 153(9):4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Hamel AF. 2014. Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. Institute for Laboratory Animal Research Journal 55(2):347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, Rosenberg K. (2014) Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized experiments 24;(83):e50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. 2014. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Frontiers in Neuroendocrinology 35(2): 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Serón-Ferré M, Hess DL, Jaffe RB. 1981. Cortisol production and metabolism in the late gestation rhesus monkey fetus. Endocrinology 108(3):916–924. [DOI] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, Ryan A, Rosenberg K, Meyer JS. 2014. Hair loss and hypothalamic-pituitary-adrenocortical axis activity in captive rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animimal Science 53(3):261–266. [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. 2013. Stress, the HPA axis, and nonhuman primate well-being: A review. Applied Animal Behavior Science 31;143(2–4):135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raul JS, Cirimele V, Ludes B, Kintz P. 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry 37(12):1105–11. [DOI] [PubMed] [Google Scholar]
- Osinski PA. 1960. Steroid 11beta-ol dehydrogenase in human placenta. Nature 187:777. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Ruppenthal GC, Elias K . 2006. Nursery Rearing of Nonhuman Primates in the 21st Century New York, Springer Science. [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. 2005. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry 57(4):373–81. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M, Rose JC, Parer JT, Foster DB, Jaffe RB. 1978. In vivo regulation of the fetal rhesus monkey adrenal gland. Endocrinology 103(2):368–75. [DOI] [PubMed] [Google Scholar]
- Sholl SA. 1983. Patterns of 3β-hydroxysteroid dehydrogenase/Δ5−4isomerase activity in the rhesus monkey placenta and fetal adrenal. Steroids 41(6):769–776. [DOI] [PubMed] [Google Scholar]
- Slikker W Jr, Althaus ZR, Rowland JM, Hill DE, Hendrickx AG. 1982. Comparison of the transplacental pharmacokinetics of cortisol and triamcinolone acetonide in the rhesus monkey. Journal of Pharmacology and Experimental Therapeutics 223(2):368–74. [PubMed] [Google Scholar]
- Slikker W Jr, Althaus ZR, Rowland JM, Hendrickx AG, Hill DE. 1984. Comparison of the metabolism of cortisol and triamcinolone acetonide in the early, mid, and late gestational age rhesus monkey (Macaca mulatta). Developmental Pharmacology and Therapeutics 7(5):319–33. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C. 2012. Analysis of cortisol in hair--state of the art and future directions. Brain, Behavior, and Immunity 26(7):1019–1029. [DOI] [PubMed] [Google Scholar]
- Sullivan E, Hinde K, Mendoza SP, Capitanio JP. 2011. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology 53(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. 1959. Evolutionary adaptation. Perspectives in Biology and Medicine 2(4):379–401. [DOI] [PubMed] [Google Scholar]
- Weinstock M 2008. The long-term behavioral consequences of prenatal stress. Neuroscience and Biobehavioral Reviews 32(6):1073–1086. [DOI] [PubMed] [Google Scholar]
- Wennig R 2000. Potential problems with the interpretation of hair analysis results. Forensic Science International 107(1–3):5–12. [DOI] [PubMed] [Google Scholar]
- Wester VL, van Rossum EF. 2015. Clinical applications of cortisol measurements in hair. European Journal of Endocrinology 173(4):M1–10. [DOI] [PubMed] [Google Scholar]
- Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, Williams MA. 2013. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Annals of Epidemiology 23(12):797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y, Teramoto M, Morimura N, Hirata S, Suzuki J, Hayashi M, Kinoshita K, Murayama M, Idani G. 2016. Analysis of hair cortisol levels in captive chimpanzees: Effect of various methods on cortisol stability and variability. MethodsX 3:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]


