Abstract
Study Objectives:
For clinicians involved in investigating and treating sleep disorders, understanding the accuracy of patient recall of supine sleep would allow informed comparisons between polysomnography (PSG) and patient-reported sleep in patients with supine-predominant obstructive sleep apnea. This study aims to assess the accuracy of patient perception of supine sleep.
Methods:
Prospective observational cohort study, assessing patient perception of total sleep and supine sleep, including duration. Data were analyzed utilizing descriptive statistics, bias-plot (Bland-Altman) analysis, and Spearman correlation (rs) to analyze relationships among continuous data.
Results:
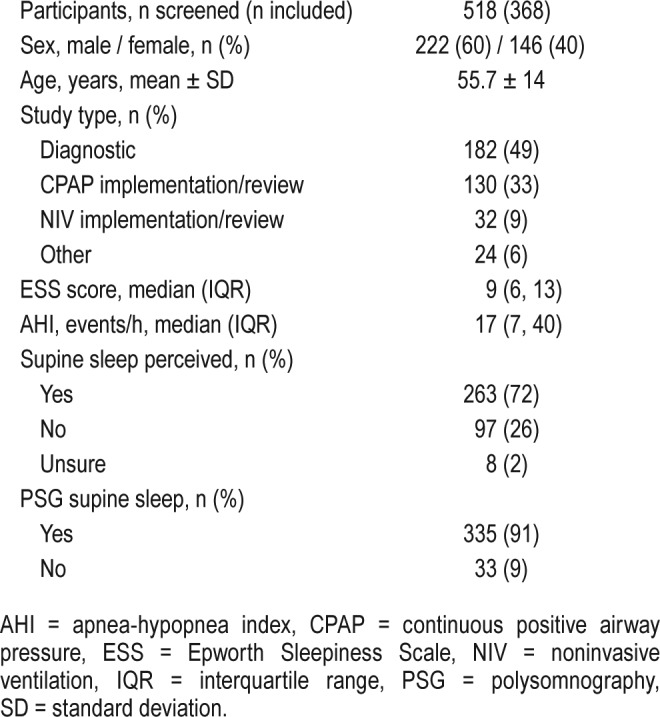
Total number of patients who underwent PSG was 518, with data from 368 of these patients analyzed. Most of these patients underwent diagnostic PSG (49.2%). Patients were excluded because of missing or incomplete data (n = 133) or immobility (n = 17). Some patients (n = 97, 26%) did not perceive supine sleep, with 34 (35% of those with unperceived supine sleep or 9% of whole group) of these having more than 60 minutes of PSG supine sleep (range 0–305.5 minutes). All “unsure” patients (n = 8, 2.2%) had significant supine sleep recorded (31.5–257.5 minutes). For the presence of any PSG supine sleep, questioning had a sensitivity of 77.9%, specificity 72.7% with positive predictive value of 96.7% and negative predictive value of 24.5%. There was a significant correlation (rs = 0.63, P < .0001) between perceived and PSG supine sleep, but wide limits of agreement (−246.9 to 194.2 minutes).
Conclusions:
In patients undergoing in-laboratory PSG, the perception of supine sleep predicts the presence of PSG supine sleep. However, questioning patients has a poor negative predictive value and patient estimates of supine sleep duration are inaccurate.
Citation:
Wallbridge PD, Churchward TJ, Worsnop CJ. Accuracy of patient perception of supine sleep. J Clin Sleep Med. 2018;14(7):1205–1208.
Keywords: accuracy, agreement, obstructive sleep apnea, patient perception, sleep-disordered breathing, sleep position
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is frequently more severe in the supine position. When discussing treatment options with patients their perception of the amount of time spent supine while asleep may affect the decision-making process, and this study aims to ascertain the accuracy of perceived supine sleep.
Study Impact: This study shows that recall of supine sleep has a good positive predictive value with a poor negative predictive value, with wide limits of agreement for duration of time spent supine. This may assist clinicians when discussing perceived usual sleep patterns with their patients.
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition characterized by repetitive collapse of the upper airway, and affects up to 25% of males in Australia.1 It has been reported to be associated with morbidity2,3 as well as increased risk of motor vehicle4 and workplace accidents.5
Supine-related sleep apnea (sometimes referred to as “positional OSA”) is typically defined as a ratio of supine to nonsupine events of greater than 2:1 and a nonsupine apneahypopnea index (AHI) of less than 5 events/h.6 Supine-related OSA commonly occurs in patients with OSA, affecting up to 60% of patients6,7 and occurring more frequently in males.8 Approximately 20% of patients with OSA have sleep apnea confined to the supine position.7 Respiratory events are also more prolonged and associated with greater desaturations while in the supine position.9 In patients undergoing in-laboratory polysomnography (PSG), spending time supine results in an increased severity of OSA, and may result in misclassification of OSA severity.10
For a clinician, patient recall of supine sleep becomes important when discussing sleep study results with patients, particularly where patients report that they do not sleep supine. Previous retrospective data suggest that patients undergoing PSG to investigate for sleep disorders poorly recall the amount of time they have spent supine in the sleep laboratory as well as position at sleep onset.11 In healthy volunteers, recall may be more accurate.12 The accuracy of recall may be important when discussing treatment options with patients and making decisions based on sleep study data and in patient acceptance of treatment.
METHODS
Consecutive adult patients (age older than 18 years) who underwent fully-attended in-laboratory PSG in a tertiary hospital in Melbourne, Australia between December 2013 and March 2014 were screened for inclusion. PSG was performed using E-Series hardware with ProfusionPSG software, version 3 (Compumedics, Abbotsford, Victoria, Australia). Position was measured using a position sensor (Compumedics E-Series part number 7000-0331-01) that was then verified and any errors amended manually, using a synchronized digital video recording. Patients completed a postsleep questionnaire regarding perception of total sleep duration and perception of supine sleep in two parts: the presence of supine sleep and estimated duration of supine sleep where supine sleep was perceived. All sleep studies were scored and staged manually for sleep stage using standard American Academy of Sleep Medicine criteria by experienced scorers. Patients with incomplete questionnaires or who had severe immobility due to underlying medical comorbidity were excluded.
Data were analyzed using descriptive statistics, bias plot analysis (Bland-Altman) to assess for limits of agreement between patient perception and PSG-recorded supine sleep, and Spearman correlation (rs) to analyze relationships between continuous data. 2 × 2 tables were utilized to assess the sensitivity, specificity, and positive and negative predictive values of perceived and PSG-recorded supine sleep. All data are mean ± standard deviation unless otherwise specified. A value of P < .05 was considered significant.
Data were entered into a spreadsheet (Excel 2007, Microsoft, Redmond, Washington, United States) and analyzed using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, California, United States).
The study protocol was approved by the Austin Health Ethics Committee (LNR/14/Austin56).
RESULTS
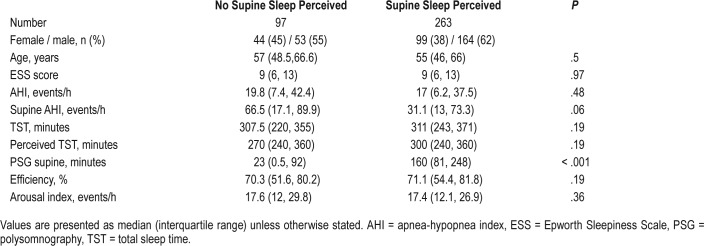
During the study period, 518 patients underwent PSG, with 150 excluded. Exclusions were due to incomplete or missing data (n = 133) or immobility caused by severe neuromuscular or neurological disease (n = 11) or spinal cord injury (n = 6). Data were considered incomplete if there was no estimation made of either the presence or duration of supine sleep. This high rate of incomplete or missing data reflects the relatively high proportion of patients from a non-English speaking background. Of the 368 included patients, the indications for sleep study were diagnostic PSG (n = 182, 49%), continuous positive airway pressure (CPAP) implementation, or treatment review for confirmed OSA (n = 130, 33%), noninvasive ventilation implementation or review (n = 32, 9%) or other indications (split diagnostic/CPAP studies, Maintenance of Wakefulness or Mean Sleep Latency Test (with preceding overnight PSG) or mandibular splint review studies, n = 24, 6%). No specific instructions regarding body position were given prior to PSG. Patient demographics and sleep parameters are recorded in Table 1.
Table 1.
Patient characteristics.
Of the 368 patients included, 263 (72%) perceived supine sleep, with only 8 (2%) unsure as to whether they had slept supine and 97 (26%) perceiving no supine sleep. A study was considered positive for supine sleep if any staged 30-second epoch of supine sleep was sampled. Overall 335 (91%) of PSG tests were positive for supine sleep with a mean duration of 140 ± 111 minutes. There was a significant difference between the duration of supine sleep sampled on PSG among those perceiving supine sleep and those who were not (Table 2). There was a moderate but significant correlation between patient perceived and PSG-confirmed supine sleep (rs = 0.63, P < .0001). Bland-Altman analysis was performed to assess the limits of agreement between perceived and measured supine sleep (Figure 1). There was a mean difference of −6.5 minutes, although the 95% limits of agreement were wide (−210.8 to 197.9 minutes), covering almost the entire duration of PSG recording. Similar results were recorded for total sleep duration (Figure 2).
Table 2.
Patient characteristics by perception status.
Figure 1. Bland-Altman plot: perceived compared to PSG-confirmed supine sleep.
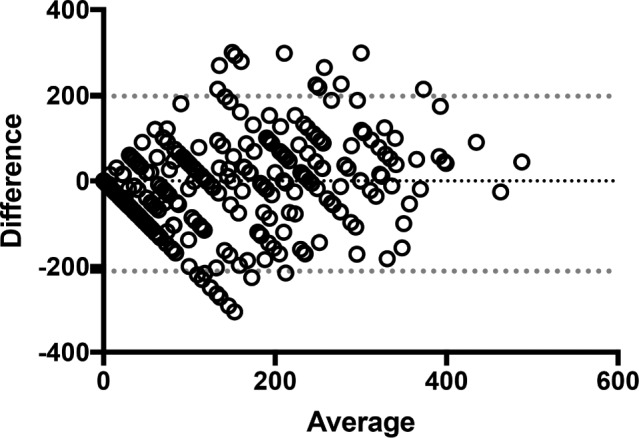
Dotted line = 95% limits of agreement. PSG = polysomnography.
Figure 2. Bland-Altman plot: perceived compared to PSG-confirmed total sleep time.
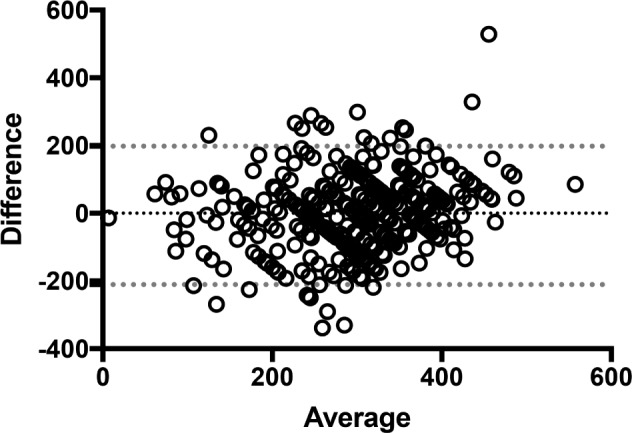
Dotted lines = 95% limits of agreement. PSG = polysomnography.
Of the 97 patients who did not perceive supine sleep, 34 (35%) had more than 60 minutes of PSG-confirmed supine sleep (range 0–305.5 minutes), with all “unsure” patients having confirmed supine sleep (range 31.5–257.5 minutes).
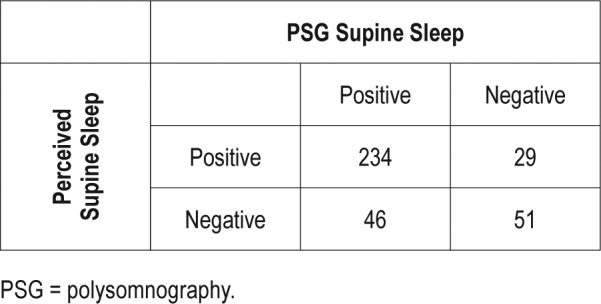
With regard to the ability of patients to perceive whether they had slept supine at all (excluding 8 unsure patients), the sensitivity for any PSG-confirmed supine sleep was only 77.4%, with a specificity of 72.4%. The positive predictive value, however, was 96.6%, with a poor negative predictive value of 24.5% (Table 3). If the threshold for considering a PSG positive is adjusted to 30 minutes of supine sleep, the sensitivity is slightly improved (83.6%) with specificity of 72.9%. The negative predictive value is improved to 52.6%, with a positive predictive value of 83.6% (Table 4). Using a threshold of 60 minutes, 34.7% of nonperceivers had supine sleep.
Table 3.
Perception compared to any PSG-confirmed supine sleep.
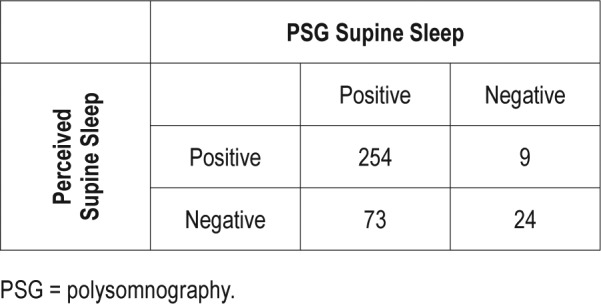
Table 4.
Perception compared to > 30 minutes PSG-confirmed supine sleep.
There were weak but statistically significant correlations identified between perceived duration of supine sleep and perceived duration of total sleep time, sleep efficiency and arousal index. There was no relationship identified between perception of supine sleep duration and other measured parameters such as AHI, sleep stage composition, or use of psychoactive medications.
DISCUSSION
In this cohort of 368 predominantly male patients with a high prevalence of sleep-disordered breathing, there was a low degree of precision, although there was a statistically significant correlation between the perception of supine sleep and PSG-confirmed supine sleep. The wide limits of agreement on assessment of agreement, spanning over 400 minutes, limit the clinical applicability of patient estimations, particularly when considering the median PSG-measured sleep duration was less than this value, at 309 minutes.
Perhaps of more clinical interest is the performance of a patient questioned regarding the presence of any supine sleep the preceding night. Our data suggest that this is probably most accurate when a patient perceives that they have slept supine, with any supine sleep sampled on PSG in 254 of 263 patients, giving a positive predictive value of 96.6%. The negative predictive value, however, is poor at 24.7%, with 73 of 97 patients who did not think they had slept supine having PSG-confirmed supine sleep. A threshold of 30 minutes of supine sleep slightly improves sensitivity (83.6 %) with similar specificity of 72.9% compared to any epoch supine. The negative predictive value is improved with a lower positive predictive value. (Table 4) Even when using a higher threshold, such as 60 minutes, more than one-third (34.7%) of nonperceivers had supine sleep. Gordon and colleagues12 found that the accuracy of recollection of sleep position in healthy volunteers was stable across two sleep studies, suggesting that this may be a generalizable finding. Given the likelihood that supine sleep will increase severity of sleep-disordered breathing, discussing the usual sleep position with patients may help ensure that diagnostic studies have not underestimated severity of sleep apnea. Unfortunately, our data suggest that the opposite situation is not true, in that a patient who spends part of the night supine in the sleep laboratory but does not think they sleep supine typically, may have a less than a 25% probability of being correct if our findings are generalizable to the nonlaboratory setting. Even when less restrictive criteria are used, the performance of a negative response remains below what may be considered useful in the clinical setting, even though awareness of the likely inaccuracy may help guide discussions regarding therapy. This scenario is common in sleep clinics, and can make discussing treatment options more difficult for clinicians, as treatments aiming to avoid supine sleep may not be accepted. Additionally, the success of supine avoidance devices that do not record sleep position may not be accurately assessed based on recall.
There are limitations with our data. First, there was a high proportion of incomplete data in our cohort, with almost 30% excluded due to missing or incomplete data. A restrictive policy was in place, however, resulting in exclusion of those without prespecified responses. Despite this, there were a broad range of patients included undergoing a variety of sleep investigations with varying degrees of sleepiness and a wide range of sleep durations. Additionally, these data are from a single-night PSG performed in a laboratory setting that may have affected sleep perception, particularly given the physical constraints afforded by PSG equipment. Finally, the population studied is from a tertiary sleep service in a large metropolitan hospital, and results may not be applicable in other settings.
CONCLUSIONS
In patients undergoing in-laboratory PSG, the ability to recall the presence or absence of supine sleep is inaccurate, with estimated duration of supine sleep showing limited agreement compared to measured supine sleep duration. This may be of importance when assessing both implementation and efficacy of positional therapy.
DISCLOSURE STATEMENT
Data from this study were presented in abstract form at Sleep DownUnder 9-11 October 2014 (Australasian Sleep Association ASM) as: 073: Accuracy of patient perception of supine sleep. Presentation of this data at Sleep DownUnder 2014 was supported by a travel grant from the Institute of Breathing and Sleep, Heidelberg, Australia. Work for this study was performed at Austin Hospital, Heidelberg, Victoria, Australia. All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Julie Tolson for assistance with initial data collection. Author contributions: PW had access to all of the data in the study and takes responsibility for integrity of the data and accuracy of analysis. PW and CW contributed to study design; PW and TC performed data collection; PW performed data analysis; PW, TC, and CW contributed to interpretation and writing of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151(5):1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Yu BY, Liu Y, Liu Y. Meta-analysis of the effect of obstructive sleep apnea on cardiovascular events after percutaneous coronary intervention. Am J Cardiol. 2017;120(6):1026–1030. doi: 10.1016/j.amjcard.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 4.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581. [PMC free article] [PubMed] [Google Scholar]
- 5.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2016;39(6):1211–1218. doi: 10.5665/sleep.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128(4):2130–2137. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 7.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 8.Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18(1):7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Leppänen T, Töyräs J, Muraja-Murro A, et al. Length of individual apnea events is increased by supine position and modulated by severity of obstructive sleep apnea. Sleep Disord. 2016;2016:9645347. doi: 10.1155/2016/9645347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiseman NA, Westover MB, Ellenbogen JM, Bianchi MT. The impact of body posture and sleep stages on sleep apnea severity in adults. J Clin Sleep Med. 2012;8(6):655–666. doi: 10.5664/jcsm.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo K, Bianchi MT. How reliable is self-reported body position during sleep? J Clin Sleep Med. 2016;12(1):127–128. doi: 10.5664/jcsm.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S, Grimmer K, Trott P. Self-reported versus recorded sleep position: an observational study. The Internet Journal of Allied Health Sciences and Practice. 2004;2(1):1–10. [Google Scholar]