Figure 2. nCDase inhibition induces a decrease of β-catenin level via phosphorylation of AKT upstream of GSK3β.
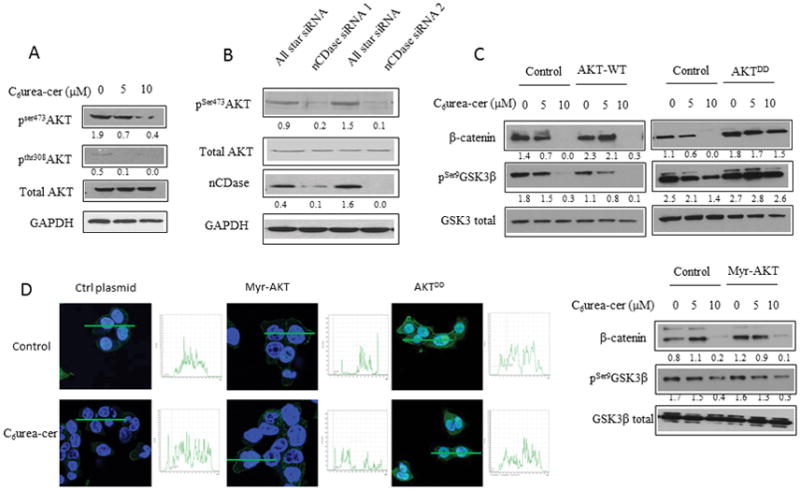
A: HCT116 cells were treated for 24 hours with the indicated concentrations of C6 urea-ceramide or with vehicle. Phosphorylation of AKT on serine 473 and threonine 308 were measured by western blot.
B: Cells were transfected twice for 48 hours with nCDase siRNA or with control siRNA. Phosphorylation of AKT on serine 473 was measured by western blot.
C: HCT116 Cells were transfected with 1μg of a plasmid encoding for a phospho-mimic constitutively active AKT (AKTDD), a wild type AKT (AKT-WT) or a myristoylated AKT (Myr-AKT) for 24 hours. Cells were treated with C6 urea-ceramide for an additional 24 hours (C6Ur-Cer) at the indicated concentrations. The total amount of β-catenin as well as phosphorylation of GSK3-β on serine 9 were measured by western blot.
D: HCT116 Cells were seeded on a glass coverslip and transfected with the indicated plasmid and then treated with C6 urea-ceramide or vehicle at the indicated concentrations for 24 hours. Cells were fixed 20 minutes in formalin, and the total AKT was detected by immunofluorescence (green). Nuclei were stained with DAPI (blue). Quantification is shown in the inset next to each image.
