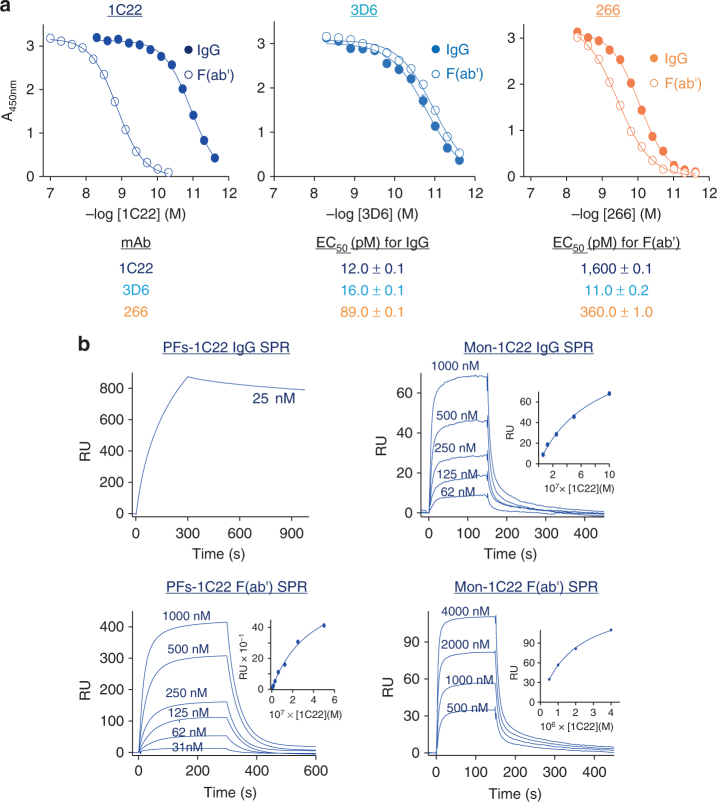
Fig. 3.
Bivalency drives 1C22 binding to PFs. a IgG and Fab binding curves for 1C22, 266, and 3D6 against plate-immobilized protofibrils (PFs). EC50 values determined from the sigmoidally fit curves demonstrated that 1C22 IgG had ~130-fold stronger reactivity against PFs than the 1C22 Fab fragment. In contrast, Fab fragments of 3D6 and 266 bound to PFs as strongly as the intact molecules. When error bars are not visible they are smaller than the size of the symbol. Values in the table are in pM and are the average ± SD of each condition analyzed in triplicate. b Representative sensograms for 1C22 IgG (upper panels) and Fab (lower panels) binding to CM5 chip-immobilized PFs (right panels) and monomer (Mon) (left panels) confirm that intact 1C22 binds more tightly to immobilized PFs than 1C22 Fab, whereas intact 1C22 and Fab bind similarly to Aβ monomer. Insets show plots of RU values at steady state for intact 1C22 and 1C22 Fab binding to PFs or Mon. The apparent binding constant of 1C22 IgG for PFs = 0.48 ± 0.002 nM, whereas the binding constant (KD) for 1C22 IgG with Mon = 1.39 ± 0.46 μM, and the KD for IgG Fab binding to PFs and Mon are: 0.80 ± 0.88 μM,1.14 ± 0.49 μM, respectively