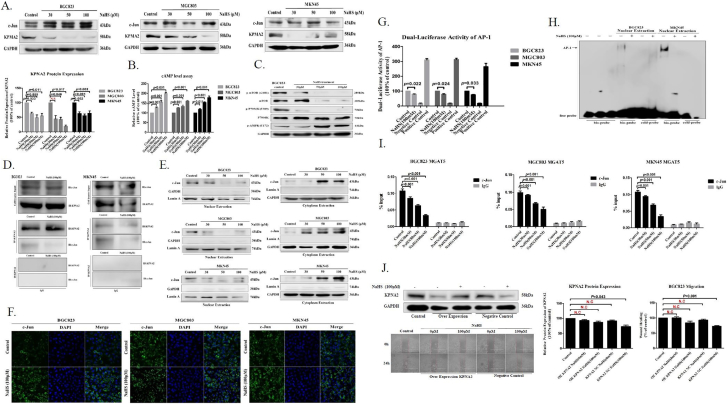
Figure 2.
H2S inhibits MGAT5 activity though specifically dissociation of KPNA2 with c-Jun interaction.
(A). After treatment with various concentrations of NaHS for 24 h, cell extracts were prepared and applied to immunoblotting with c-jun and KPNA2. GAPDH was used as a loading control. (B). The cAMP activity was assayed by HTRF. Various concentrations of NaHS were added to GC cells. (C). Western blotting of protein on the mTOR signaling pathway in BGC823 cells after 24 hours with NaHS treatment indicated various concentrations. GAPDH was used as a loading control. (D). Co-Immunoprecipitation detection the relationship between KPNA2 and c-Jun with NaHS treatment after 24 hours in BGC823 and MKN45. (E). Western blotting assayed c-Jun from nuclear and cytoplasmic extracts of GC cells treated with various concentrations for 24 hours. Lamin A and GAPDH was used as a loading control. (F). Immunofluorescence staining of c-Jun in GC cells treated with NaHS at 100 μM after 24 hours. (G). Luciferase reporter assay measuring AP-1 activity in GC cells transiently co-transfected with pAP-1-Luc with NaHS treatment for 24 hours at 100 μM. (H). EMSA assay of DNA binding to AP-1 in the nuclei extracts from BGC823 and MKN45 cells after NaHS treatment for 24 hours at 100 μM. Results are repetitive from at least two independent experiments. (I). GC cells were incubated with NaHS and analyzed by a quantitative ChIP assay with anti-c-Jun antibody. (J). The inhibitory effect of H2S on migration of serum free stimulated stably KPNA2 over-expression in BGC823. Each bar represents the means ± S.D. of three independent experiments.