Abstract
Background: In many countries, there are growing numbers of persons living with a prior diagnosis of cancer, due to the aging population and more successful strategies for treatment. There is also growing evidence of the importance of healthful diet and weight management for survivorship, yet many long-term cancer survivors are not successfully following recommendations. Methods: We explored this issue in a mixed methods study with 53 adult survivors of 3 cancers (breast, prostate, and non-Hodgkin’s lymphoma), living in Maryland. Participants provided three 24-hour dietary recalls, and results were used to classify respondents on 2 metrics of healthful eating (the Healthy Eating Index 2010, and a 9-item index based on current dietary recommendations). Recalls were also used to guide in-depth qualitative discussions with participants regarding self-assessment of dietary behaviors, healthful eating, and diet’s importance in cancer prevention and survivorship. Results: Survivors following a more healthful diet were more likely to be female, have greater socioeconomic resources, more years since diagnosis, normal weight, and no smoking history. Qualitative discussions revealed a more nuanced understanding of dietary strategies among healthful eaters, as well as the importance of household members in dietary decision making. Discussion: Most survivors had received little nutrition counseling as part of their cancer care, highlighting the importance of holistic, household-oriented nutrition education for maintaining health among long-term cancer survivors.
Keywords: mixed methods, dietary assessment, cancer survivorship, nutrition education, clinician behavior
Introduction
In the United States and many Western countries, the number of adults living with a history of cancer continues to grow, driven by both the aging of the population, as well as increased likelihood of successful treatment and survival.1,2 The US lifetime risk of cancer is now 41% for men and 38% for women,3 but the 5-year relative survival rate for those diagnosed between 2006 and 2012 was 67%, up from 49% for those diagnosed between 1975 and 1977.4 The term silver tsunami is used to describe the growing numbers of older survivors; of 15.5 million US survivors in 2016, 62% were 65 years or older.5
As survival rates increase, so does evidence that individual health behaviors after treatment, including diet, physical activity, and a healthful weight, contribute to reducing recurrence risk, and maintaining health and well-being.6-8 Although the diversity of biological drivers of each cancer type is more widely recognized, so too is the evidence supporting a broad role for health behaviors across many cancer sites and survivor populations. For breast cancer, a review of 85 studies6 found indications of improved survivorship with moderate physical activity, healthy body weight, and reduced fat, higher fiber diet. For prostate cancer, evidence supports associations between both weight gain7 and obesity8 and risk for recurrence after treatment, and all-cause mortality.
Arguably as important as decreasing risk for cancer recurrence, a healthful lifestyle can also help cancer survivors cope with treatment-related symptoms, maintain physical function and psycho-social well-being, and reduce risk for other chronic diseases including diabetes and heart disease.9 For some cancers, survivors experience increased risk for chronic comorbidities due to their cancer or treatment effects. Additionally, for many cancer patients, their prediagnosis patterns of tobacco use, poor diet, overweight, or inactivity, which put them at great initial risk to develop cancer, also increase the likelihood they will experience additional chronic health problems during survivorship.
Although the initial cancer diagnosis is often seen as a “teachable moment,” and interventions with recent survivors demonstrate short-term success, most data suggest that adult long-term cancer survivors are no more likely to maintain a healthful diet than nonsurvivors of similar age, gender, ethnicity, and socioeconomic backgrounds.10-12 However, findings also suggest that cancer survivors actively seek guidance on effective strategies to improve their cancer course and avoid recurrence.13 One reason for a lack of long-term success, despite motivation among survivors to remain healthy after treatment, is that, especially for older adults, changing dietary behaviors established over a lifetime is challenging, and requires tailored support over time to maintain behavior changes.
It is well recognized that cancer care providers are not always equipped to educate or support survivors regarding dietary choices and behavior change strategies.14 Nutrition-related behavior change is best addressed by an ecological framework, to understand and potentially intervene on individual, family and household, and community-level influences on dietary behaviors. To move beyond broad dietary assessments and identify potential avenues for dietary intervention, formative research is useful to explore cancer survivors’ perspectives on healthful diet, as well as influences in their daily lives that serve as promoters or barriers to dietary change.
Methods
Study Overview
The Eating for Life study, funded by the National Cancer Institute, was a 2-phase exploratory study to examine diet-related attitudes and behaviors among survivors of 3 types of cancer: female breast, prostate, and non-Hodgkin’s lymphoma (NHL). These 3 cancers were selected due to their overall favorable survival rates, as well as their ability to offer comparisons by gender, strength of evidence regarding diet in survivorship, and intensity and duration of treatment (to maximize variation in contact with providers).
The first phase of the study collected in-depth interview data regarding their perspectives on nutrition and survivorship from a range of cancer care providers.15-17 In the second phase, a mixed methods concurrent design was used to explore dietary beliefs and behaviors among survivors themselves, using structured dietary recalls for dietary assessment, and as tools for participant reflection and qualitative discussions.18-20 A mixed methods approach to understanding dietary behaviors synthesizes evidence from both structured data on specific dietary components and qualitative data describing contextual and cultural aspects of eating, to provide a fuller understanding of this complex behavior.21
Here, we focus specifically on patterns of healthful diet among survivors, and also compare characteristics of those reporting relatively healthful dietary patterns to those with less healthful diets. As well, we explore the concept of “healthful diet” with survivors themselves, and the extent to which their own explanatory models of diet are congruent with recommendations. The goal of the analysis is to identify specific dietary elements and behaviors, as well as broader thematic areas of challenge with regard to nutrition behaviors, which might be targets for nutrition education interventions.
Recruitment and Data Collection
During a 12-month period in 2012-1013, we recruited a total of 53 adult survivors of breast, prostate and NHL cancers in the Baltimore-Washington area, through direct chart review and physician referrals at two hospital-based cancer centers (one academic and one community-based), as well through support groups and word of mouth. Inclusion criteria specified English-speaking participants aged 45 to 74 years, 3 or more years postdiagnosis, with completed acute treatment, and no known current metastases or recurrent cancer. A purposive sampling methodology22 was used to capture variation in time since diagnosis, ethnicity and race, age, and cancer site, to maximize ability to explore the influence of these factors on dietary attitudes and behaviors. Twenty-one men and 32 women participated.
During an in-person appointment at the research offices, participants completed a structured staff-administered questionnaire about health and sociodemographic characteristics, an audio-recorded in-depth interview, and a staff-guided computer-based Automated Self-Administered 24-Hour (ASA-24) dietary recall for the previous day.23 The in-depth interview guide focused on 6 thematic areas: identity issues, survivorship history (diagnosis, treatment, adjustment to life after treatment), assessment of health status, impact of cancer on social and family relationships, and cancer in the context of daily life (return to work and other important aspects of life). Subsequently, project staff contacted participants on 2 additional days (in a 14-day window) without prior notice to ask them to log into the ASA-24 system and complete 24-hour recalls, providing 2 weekday and 1 weekend recalls (two participants lacked internet access; staff collected recalls by telephone). Nonsequential 24-hour recalls were selected as the preferred method for assessing the dietary components of interest (see below) and for examining daily patterns of food preparation and intake.24,25 A final office-based audio-recorded in-depth interview occurred 1 to 2 weeks after the last recall. Participants were given their individual recall-derived summaries for 9 specific dietary components, as well as recommended values, and this recall-based summary served as a discussion tool for comparing current and aspirational dietary practices. The in-depth interview guide focused on typical meal patterns, social, familial, and environmental context for food purchasing and consumption, dominant foods in the diet, diet and eating before cancer diagnosis, thoughts about foods and diets in relation to cancer, diet in relation to any efforts to control weight or eat healthy, and barriers to improving or controlling diet, including the role of health care providers. All participants provided informed consent, and received $140 for participation in all data collection. The study was approved by the Johns Hopkins Institutional Review Board.
Measurement and Data Analysis
Structured interview components included self-reported age, ethnicity and race, marital status, household size, years of education, current work status, and categories of household income. Two health measures were used: the 12-item Short Form Health Survey (SF-12)26, which is used to calculate a well-validated population-normed assessment of perceived physical and mental well-being, and a self-reported list of 12 common chronic illnesses (arthritis/rheumatism, diabetes/high blood sugar/sugar in urine, inflammatory bowel disease/colitis/Crohn’s disease, bleeding from stomach ulcers, chronic lung disease/bronchitis/emphysema, heart failure/congestive heart failure, stroke/brain hemorrhage, hypertension/high blood pressure, myocardial infarction/heart attack, angina/chest pain, cirrhosis/liver disease, depression/anxiety). Additional health indicators included body mass index (BMI), converted from self-reported height and weight (BMI = weight in pounds/(height in inches × height in inches)) × 703), smoking status, and weekly minutes of moderate activity.27
Calculation of the Healthful Dietary Score
ASA-24 recall data are collected directly through a secure website maintained by the National Cancer Institute, which returns analytical files to researchers containing both reports of meals, foods, and specific item intake, as well as dietary nutrients and other components. In addition to the nutrient analysis data, we used daily recalled intake reports for qualitative textual analysis of participant eating characteristics.
We analyzed the structured ASA-24 data by calculating 2 different summary scores, in order to explore cancer-related dietary behaviors, as well as compare our respondents to broader populations in regard to general dietary recommendations. First, averages were calculated across the three 24-hour recalls for 9 dietary components, based on broadly accepted dietary recommendations to prevent cancer and other chronic diseases,28,29 and assigned a value of 0 or 1 to each respondent, based on meeting recommendations. The 9 dietary behaviors included no more than moderate intake of five unhealthful dietary components, including alcohol intake (≤1 drink daily for women, ≤2 for men), cholesterol (<300 mg), total calories (1600-2200, based on age, gender, and sedentary/active lifestyle), saturated fat (≤10% of total calories), and sodium (≤2300 mg, or ≤1500 for age 50 years and older, African Americans, or those with specific chronic diseases), and sufficient intake in 4 domains, including fruits and vegetables (5+ servings), vitamin D (≥15 µg, ≥20 if age 70+ years), calcium (≥1000 mg if age 31-50 years, ≥1200 mg if age 51+ years), and fiber (≥25 g for women, ≥28 g for men). Participant scores of 0 or 1 on the nine elements were summed for a composite score, indicating number of recommendations met. In addition, the Healthy Eating Index 2010 (HEI-2010) score was also calculated, based on 12 components: adequate intake of total fruit, whole fruit, total vegetables, green vegetables and beans, whole grains, dairy, total protein foods, seafood/plant proteins, moderate intake of refined grains, sodium, so-called empty calories—added sugars, solid fats, alcohol, and an appropriate ratio of unsaturated to saturated fats.30 The purpose of these indices was not as formal cut points for healthful or unhealthful diets, but rather as tools for data reduction across multiple dietary elements, and as one way to compare and contrast behaviors and perspectives across participants.
Qualitative Data Management and Analysis
Audio-recordings of in-depth interviews were transcribed verbatim, and 2 team members coded for thematic content using ATLAS.ti software (www.atlasti.com). For this analysis, which focuses on identifying characteristics and potential drivers of healthful and less healthful dietary behaviors, a constant comparative approach31 was used to compare within and across all participants, including particular focus on contrasting persons with higher scores on the dietary indices to those with lower scores. Qualitative analyses were framed around 4 broad thematic topics and questions:
How did the foods, meals and eating patterns of high and low scorers differ qualitatively?
How did high and low scorers respond to the feedback they received on their recalls? How did this information fit with what they knew, or believed, about their diets?
How did all participants talk about their family and significant others’ influence on their efforts to eat healthfully?
What do all survivors say that they need to live as a “healthy” survivor, and what avenues for intervention are suggested by the results?
Results
Structured Data Results
Table 1 describes the current dietary patterns of the participants, based on analyses of their ASA-24 recalls. Two of the 9 dietary goals appeared to be commonly met: 89% of these cancer survivors stayed within general adult guidelines for limiting alcohol consumption, and two-thirds reported 5 or more fruit and vegetable servings per day. However, 5 of the recommendations were met by only about half of the respondents (cholesterol 55%, total calories 51%, vitamin D 53%, calcium 49%, and saturated fat 40%). Two recommendations were especially challenging for this population to meet. Only 15% of respondents consumed adequate fiber across their 3 recall days, and only 2% reported sodium consumption within recommended limits. Summed index scores for respondents ranged from 1 to 8, indicating that all respondents met at least one dietary recommendation, but no respondent achieved all nine. The median number of recommendations met was 4. The HEI reflects a similar diversity among participants. Scores ranged from a low of 36 (indicating many areas of poor nutrition) to 86, with an average value of 63 (similar to the US population average of 59). Among these 53 individuals, index scores and HEI scores were positively and moderately correlated (r = 0.49, P < .001). Table 1 also displays the distribution of each indicator by cancer type. For the most part, breast cancer and NHL survivors reported healthier eating patterns than prostate cancer survivors.
Table 1.
Healthful Dietary Behaviors of Cancer Survivors (Based on Three 24-Hour Recalls).
| Healthful Dietary Behaviora | All Respondents (n = 53) |
Breast Cancer (n = 25) |
Prostate Cancer (n = 20) |
Non-Hodgkin’s Lymphoma (n = 8) |
|---|---|---|---|---|
| % Meeting Goal | % Meeting Goal | % Meeting Goal | % Meeting Goal | |
| Avoids alcohol | 89 | 92 | 85 | 88 |
| Adequate fruits and vegetables | 66 | 72 | 65 | 50 |
| Limits cholesterol | 55 | 68 | 35 | 63 |
| Healthful range of total calories | 51 | 48 | 45 | 88 |
| Adequate vitamin D | 53 | 56 | 45 | 63 |
| Adequate calcium | 49 | 52 | 40 | 63 |
| Limits saturated fat | 40 | 52 | 30 | 25 |
| Adequate fiber | 15 | 24 | 5 | 13 |
| Limits sodium | 2 | 4 | 0 | 0 |
| No. of Recommendations Met | % of Respondents | % of Respondents | % of Respondents | % of Respondents |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 4 | 4 | 5 | 0 |
| 2 | 13 | 4 | 25 | 12.5 |
| 3 | 15 | 12 | 20 | 12.5 |
| 4 | 26 | 28 | 25 | 25 |
| 5 | 21 | 20 | 20 | 25 |
| 6 | 13 | 20 | 5 | 12.5 |
| 7 | 6 | 8 | 0 | 12.5 |
| 8 | 2 | 4 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 |
| Healthy Eating Index (HEI) Scoreb | % of Respondents | % of Respondents | % of Respondents | % of Respondents |
| 36-48 | 21 | 8 | 40 | 12.5 |
| 51-64 | 24 | 20 | 30 | 25 |
| 65-74 | 36 | 44 | 20 | 50 |
| 75-86 | 19 | 28 | 10 | 12.5 |
Based on 2010 Dietary Guidelines or other cancer-relevant recommendations: Alcohol, <1 drink/day for women, <2 for men; fruits and vegetables, 5+ servings/day; cholesterol, <300 mg; kcals, 1600-2600, by age, gender, sedentary versus active; vitamin D, >15 µg (>20 µg if age 71+ years); calcium, 1000 mg (age 31-50 years), 1200 mg (age 51+ years); saturated fat, <10% calories from saturated fat; fiber, 25 g for women, 28 g for men; sodium, <2300 mg (<1500 mg for 50+ years, African American, chronic illness).
Healthy Eating Index 2010—scored 0-100, 12 scored components: Total Fruit; Whole Fruit (not juice); Total Vegetables; Greens and Beans (dark-green vegetables, beans, peas); Whole Grains; Dairy (milk products, soy beverages); Total Protein Foods; Seafood and Plant Proteins; Fatty Acids (ratio of poly- and mono-unsaturated to saturated fat); Refined Grains; Sodium; Empty Calories (calories from solid fats and added sugars, calories from alcohol beyond a moderate level). Higher scores for greater intakes, except sodium, refined grains, empty calories.
Table 2 displays participant sociodemographic and health characteristics, and compares participants’ number of recommendations met and HEI scores by key characteristics. Despite the limited sample size, many patterns are observed.
Table 2.
Bivariate Comparisons of Healthful Diet by Characteristics of Sample Participants (n = 53).
| Participant Characteristics | % of Sample | Average Number of Recommendations Met | Average Healthy Eating Index Score |
|---|---|---|---|
| Demographics | |||
| Age range, years | |||
| 47-54 | 28 | 4.4 | 67.4* |
| 55-74 | 72 | 4.1 | 61.2 |
| Gender | |||
| Male | 40 | 3.5*** | 56.6*** |
| Female | 60 | 4.7 | 67.1 |
| Race/ethnicity | |||
| White | 60 | 4.3 | 63.8 |
| Black | 36 | 3.8 | 60.8 |
| Asian or Pacific Islander | 4 | (too few to include) | |
| Marital status | |||
| Married/living as married | 60 | 4.3 | 63.1 |
| Not currently married | 40 | 4.0 | 62.8 |
| Household size | |||
| Lives alone | 23 | 4.5 | 68.9* |
| Lives with others | 77 | 4.1 | 61.2 |
| Education | |||
| High school/Technical | 19 | 3.0*** | 51.7*** |
| College or graduate degree | 81 | 4.5 | 65.6 |
| Occupational status | |||
| Currently working | 51 | 4.6* | 65.5 |
| Not currently working | 49 | 3.8 | 60.3 |
| Household income, $ | |||
| <50 000 | 21 | 3.1** | 55.2* |
| ≥50 000 | 71 | 4.5 | 65.4 |
| Don’t know/refused | 8 | (too few to include) | |
| Cancer experience | |||
| Cancer type | |||
| Breast | 47 | 4.7** | 67.6** |
| Prostate | 38 | 3.5 | 56.9 |
| Non-Hodgkin’s lymphoma | 15 | 4.5 | 63.9 |
| Age at diagnosis, years | |||
| 33-59 | 62 | 4.2 | 65.3* |
| 60-70 | 38 | 4.1 | 59.0 |
| Years since diagnosis, years | |||
| 2-4 | 49 | 3.7** | 60.6 |
| 5-24 | 51 | 4.7 | 65.2 |
| Health and well being | |||
| SF-12 Physical Health subscalea | |||
| 17.6-49.9 | 31 | 4.0 | 62.6 |
| 50.0-65.6 | 69 | 4.3 | 63.4 |
| SF-12 Mental Health subscalea | |||
| 27.2-49.9 | 15 | 4.8 | 62.4 |
| 50.0-65.6 | 85 | 4.1 | 63.3 |
| No. of comorbidities (of 12 common chronic conditions)b | |||
| None, or 1 | 53 | 4.1 | 66.4 |
| 2 or more | 47 | 4.3 | 62.2 |
| Body mass index (BMI) | |||
| Underweight/Normal weight | 32 | 4.9** | 69.7 ** |
| Overweight/Obese | 68 | 3.9 | 59.8 |
| Tobacco use history | |||
| Current/Former smoker | 38 | 3.5*** | 60.5 |
| Never smoked | 62 | 4.6 | 64.5 |
| Current physical activity level | |||
| <150 min weekly (moderate intensity) | 45 | 4.0 | 62.8 |
| ≥150 min weekly (moderate intensity) | 55 | 4.4 | 63.0 |
Based on complete responses from 52 participants.
Whether respondents had been told by a doctor that they had: arthritis or rheumatism, diabetes/high blood sugar/sugar in urine, inflammatory bowel disease/colitis/Crohn disease, bleeding from stomach ulcers, chronic lung disease/bronchitis/emphysema, heart failure/congestive heart failure, stroke/brain hemorrhage, hypertension/high blood pressure, myocardial infarction/heart attack, angina/chest pain, cirrhosis/liver disease, or depression or anxiety.
Trend toward statistically significant difference in means, P < .10.
Statistically significant difference in means, P < .05.
Statistically significant difference in means, P < .01.
Sixty percent of respondents were pre-retirement age (<65 years), and 60% were women. Reflecting the recruitment area demographics, 60% were white, and 36% were African American. Sixty percent were living with a spouse or partner. Most participants had at least one other member of their household, although 23% lived alone. The participants had considerable social and economic resources, in that 81% had at least a college degree, and of those disclosing income, most had household incomes of $50 000 or more. Half were currently working.
We recruited almost equal numbers of breast and prostate cancer survivors, but fewer NHL survivors as participants. Age at diagnosis ranged from 33 to 70 years, providing an important diversity across the life course for the cancer experience. Almost half of respondents had experienced their diagnosis within the past 2 to 4 years, but some respondents were as much as 24 years from diagnosis.
Overall, participants reported good perceived physical health and even better mental health, with 69% scoring at or above the SF-12 physical health midpoint, and 85% scoring at or above the mental health midpoint. Nevertheless, 47% reported 2 or more chronic disease or conditions (in addition to cancer), and 68% of participants were overweight or obese. The majority of participants had never smoked, 34% were former smokers, and only 4% smoked currently. About half of respondents met physical activity recommendations.
Younger participants trended toward higher average HEI scores (P < .10) but did not differ on number of recommendations met. No differences were seen between white and black participants, but well-known differences in dietary behaviors between men and women were present, with women averaging almost 10 points higher on HEI score, and meeting 1.2 more recommendations. Similarly, well-recognized differences based on socioeconomic resources were observed. Participants achieving college or graduate education had higher HEI scores and met more dietary recommendations, and higher income participants met significantly more recommendations, and trended toward higher HEI scores. Two additional trends (P < .10) were higher HEI scores among respondents who lived alone, and a larger average number of recommendations met by those currently working.
Differences by cancer type were similar to those observed by gender, with breast cancer survivors (and to a lesser degree, NHL survivors) meeting more dietary recommendations and having higher HEI scores than prostate cancer survivors. Survivors at least 5 years postdiagnosis ate more healthfully than those with more recent diagnoses, and a trend was observed toward higher HEI scores among those diagnosed at a younger age.
Eating habits did not vary significantly by mental or physical self-assessed health, number of chronic conditions, or regular moderate exercise. Overweight and obese survivors reported less healthful diets than normal or underweight participants, and current or former smokers met fewer dietary recommendations than never-smokers.
Qualitative Findings
Qualitative results are discussed below along the four main thematic areas described above. Quotes were selected to illustrate each theme and subtheme identified.
1. How did the foods, meals and eating patterns of high and low scorers differ qualitatively?
Survivors who reported more healthful diets (meeting 6-8 of 9 recommendations, n = 11) shared common characteristics in their three 24-hour recall days. They tended to “graze,” eating 2 rather than 3 meals, adding multiple small snacks throughout the day. This pattern also carried over into formal meals, where they were more likely to “mix their plate” by adding small portions of a variety of foods, including fruit, crackers, and vegetables. They were less likely to eat processed or pre-prepared foods, or, if they did so, eat them only in moderation. They also reported more meals and days with little or no meat consumption, compared to survivors whose scores were lower. Despite their overall healthful patterns, their recalled daily diets were often low in fiber, and high in sodium.
In comparison, some of the participants scoring only 1or 2 on the 9-item index (n = 8) were more likely to eat so-called “three squares a day,” consuming larger meals that featured a meat-based entrée, including at breakfast and lunch. This pattern was especially common for men. Snacking between meals, typically with cookies or chips, was more common, as was reporting pre-prepared or carry-out meals. Vegetables were typically reserved for evening meals as a single cooked side portion. A subgroup of these poorly scoring survivors, primarily women, reported eating on an altered schedule, due to shift work or other external rhythms in their lives. For those participants, meals eaten late at night often featured pre-prepared foods.
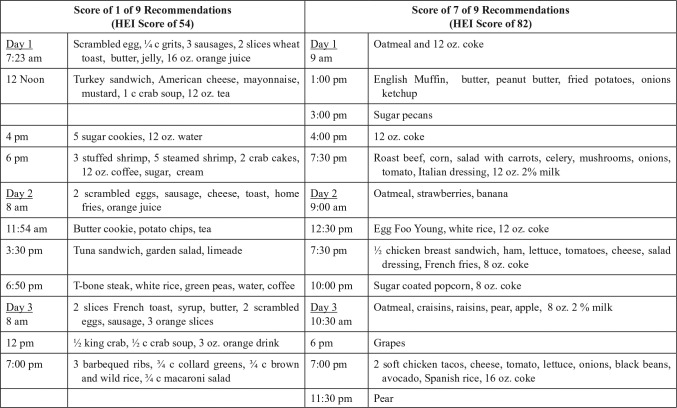
As illustrative examples, Figure 1 presents recalls from 2 women who were breast cancer survivors: a 51-year-old achieving only 1 recommendation and having an HEI score of 54, and a 54-year-old meeting 7, with an HEI score of 82. Notably, the participant scoring poorly had many nutritious elements in her diet, and the participant scoring better nevertheless reported many unhealthful dietary behaviors, including ≥16 ounces of sweetened cola on each recall day.
Figure 1.
Comparison of 24-hour recalls for a low- and a high-scoring participant.
2. How did high and low scorers respond to the feedback they received on their recalls? How did this information fit with what they knew, or believed, about their diets?
Figure 2 compares 5 themes regarding dietary beliefs from individuals who had lower scores, to four themes emerging from those reporting more healthful diets. Overall, persons with less healthful diets were more likely to discount their ability to improve it, either because healthful diets were unpleasant and arduous to follow, or because their cancer course had made it too challenging. In contrast, those eating more healthfully reported multiple small strategies taken to maintain healthful diet and weight. Low scorers tended to focus on personal weakness (“sloth, indulgence, cravings, and temptations”) whereas healthful eaters had a more nuanced understanding of environmental cues, and named effective strategies, such as preparing food in advance, and bringing healthful food to breakfast meetings.
Figure 2.
What is a healthy diet and how is it achieved?
3. How did family and significant others influence high and low scorers’ efforts to eat healthfully?
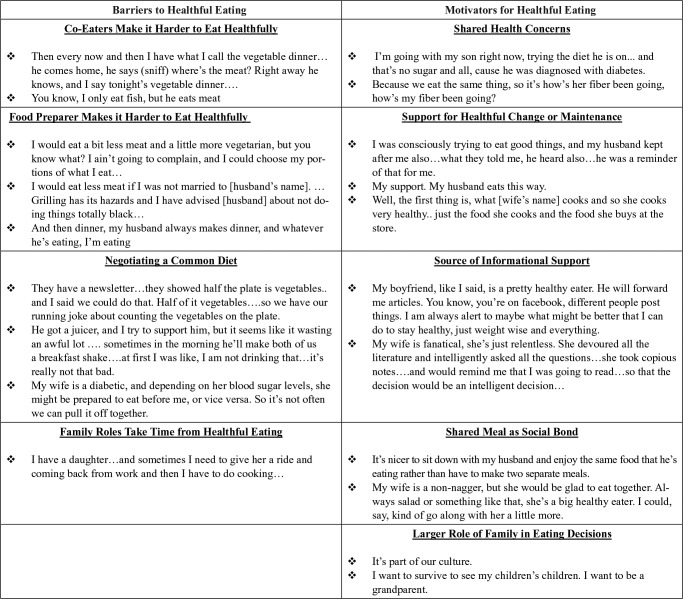
Seventy-seven percent of participants lived with at least one other person, and discussions demonstrated that family members and significant others served as both facilitators of, and deterrents to, healthful eating. Figure 3 describes 5 themes related to how family and significant others served as motivators for healthful eating (with illustrative quotes) and 4 themes related to barriers to healthful diet.
Figure 3.
How does the family influence healthful eating?
For both high and low scoring survivors, family member attitudes toward food played a significant role in their own eating patterns. Many women reported that their husband or partner was the primary cook, and that changing their own diet was contingent on persuading their partner to accept changes. Others reported that another household member’s dietary needs, for example due to chronic disease, could present either an additional planning challenge, or alternatively, additional support for healthful household behaviors.
4. What do survivors feel they need to live as a “healthy” survivor, and what avenues for intervention are suggested by the results?
The Nature of Nutrition Education
Despite a general sense that nutrition information was widely known, most respondents were surprised by 24-hour recall results. Most respondents were unaware they routinely exceed recommended sodium levels and were concerned to learn their fiber intakes were below recommended levels.
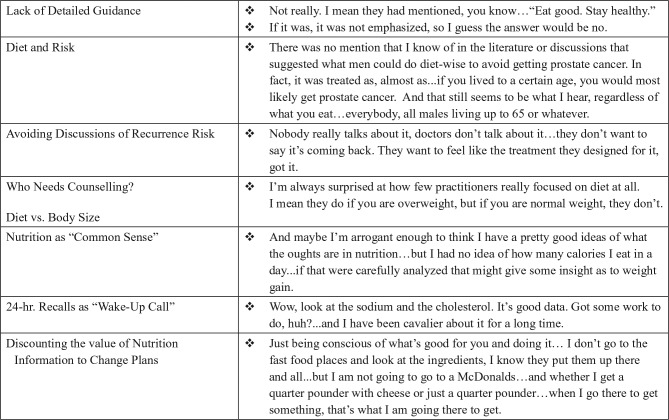
Furthermore, as the quoted “Negative Views” in Figure 2 and “Barriers” in Figure 3 describe, many respondents expressed beliefs that nutrition behaviors were challenging to maintain, despite general nutrition knowledge. Participants reflected that nutrition behavior change was not simply knowing what one should or should not eat, but how to modify one’s nutrition behavior to maintain healthful diets (Figure 4).
Figure 4.
Nutrition education experiences and perspectives.
The Limited Role of Clinicians in Guiding Survivorship Diet
Across all survivors, a common theme was a lack of detailed guidance from clinicians regarding diet, especially after active treatment had ended. Survivors often had little knowledge of links between diet and cancer, and even less understanding of the potential role of diet in reducing risk for recurrence. Respondent statements about dietary discussions with providers are illustrated in Figure 4.
Conclusions
Dietary Patterns Among Cancer Survivors
In examining the dietary behaviors of this diverse group of cancer survivors, it is noteworthy that our small sample reflects patterns of diet consistent with both national data on adult cancer survivors, as well as the general adult population. Despite the “life-altering” experience of cancer diagnosis and treatment, survivors’ eating behaviors reflect the common day-to-day food culture shaping all US diets, including widespread availability of inexpensive, appealing foods high in fats, sugars, and sodium, and low in fiber and other nutrients.32 As well, findings are consistent with other dietary studies, which identify subgroups (including persons of lower socioeconomic status, men, and persons who are overweight or have histories of tobacco addiction) as most in need of dietary improvement.33
What this more in-depth study of survivors contributes is the ability to link these dietary behaviors with participant reflections and interpretations. Somewhat paradoxically, as Figure 2 shows, many survivors who scored relatively poorly on dietary quality (1-2 of 9 recommendations met) nevertheless believed their diets to be generally healthful, while higher scorers (6-8 recommendations met) were more likely to describe additional goals or areas for work in their eating behaviors. Some respondents reported making healthful dietary changes, describing the cancer experience as a “wake-up call” or “second chance to lead a healthful life.” However, an equal number reported “letting go” of dietary restrictions, either to enjoy more fully a life they now saw as finite, or rejecting health rules they had once lived by, which had not protected them from cancer. For the most part, survivors with poor diets had little knowledge of diet’s role in risk for recurrence, and, as discussed below, had little guidance from clinicians about dietary strategies to promote healthful survivorship.
Limitations
These findings are based on cross-sectional data, examining dietary patterns at a single point in time during the cancer trajectory. This study by design focused on perceptions of dietary change, rather than actual eating patterns before and after cancer events. Despite the relative frequency of cancer in the general population, it would be challenging to design a cohort study large enough to prospectively capture dietary behaviors across a long period, allowing comparisons of dietary changes among cancer survivors to those occurring during “normal” (ie, cancer-free) aging.
As well, this project was designed to focus deeply on a relatively small number of survivors. Although some potentially informative bivariate patterns were observed, such as statistically significant differences by time since diagnosis and household composition, these relationships merit confirmation in larger samples, to rule out potential confounders. Moreover, although sampling purposefully maximized diversity in terms of cancer type, academic versus community care setting, urban (and less affluent) versus suburban or rural residence, and ethnic background, these survivors were well-resourced for the most part, and had received comparatively high-quality treatment for their cancer. Thus, findings may be conservative in terms of unaddressed needs, which are likely even more pronounced in lower resource survivor populations. However, issues among these survivors are likely highly salient across all survivor groups.
Influences on Diet and Avenues for Intervention
A second set of findings from this study examine the ecological influences on dietary behavior among survivors, to explore areas for potential intervention. Of the many influences on eating, this analysis focused on two of the arguably most important—the day-to-day influence of family and household, as well as the more sporadic but highly influential role of guidance from medical care providers.
Findings reinforce that family and household members play influential roles in most cancer survivors’ diets. Even in households where women maintain traditional food-related roles, spouses and partners, as well as adult children and grandchildren, have significant influence, both as facilitators and barriers to healthful diet.
Furthermore, many households showed substantial influence from men as cooks and shoppers, beyond their typical role as food consumers. This could be due to shared household responsibilities during the cancer experience, or other transitions, such as retirement after cancer. In addition to dietary needs of the cancer survivor in the household, health concerns of other family members, such as weight loss or other chronic illnesses, were also influential. Interventions involving food planners, purchasers and preparers are more likely to be successful if they strive to address the household’s diet, rather than just the survivor’s diet.34
With regard to the influence of health care providers on survivor diets, findings reflect those from a 2005 review, suggesting that insufficient progress has been made over the past ten years. There is an urgent need for clinicians to more seriously address nutrition posttreatment, to meet the population-level need for healthful survivorship. Increasing numbers of persons experiencing a diagnosis of cancer, and advances in treatment will produce more survivors, and the nutrition transition means more of those survivors will bring unhealthful dietary behaviors to their cancer experience. Thus, cancer clinicians may not be the first health care professional to engage these patients in discussions of dietary improvement, but they have a unique opportunity to play an influential role, if they can successfully leverage the “teachable moment” offered by cancer during the mid and late adulthood health-related life course.
The role of health care providers in supporting nutrition behavior change has 2 important components. There is a need for the delivery of information, to persuade survivors of the evidence connecting healthful diet to longer and better quality survivorship trajectories. As well, there is the need to offer support for behavior change, drawing on the well-established evidence base for clinician-supported behavior change in areas such as tobacco cessation.
This study’s first phase demonstrated that many clinicians involved in caring for survivors require additional education for both these tasks.15 Some clinicians are not persuaded that the evidence is sufficiently strong to promote diet and weight loss as survivorship strategies, suggesting a need for broader dissemination of current consensus reports.
However, the more common barrier to nutritional counselling in the cancer care setting is a lack of role identification for the task,17 and skill in the delivery of interventions such as brief motivational counseling. Many providers who understood the importance of weight control nevertheless expressed a sense of personal futility, as a clinician, about intervening on patient dietary behaviors, citing time constraints, infrequent patient contact, and inadequate knowledge and skills in effective intervention approaches.
These survivor discussions demonstrate that patients vary widely on their awareness and readiness to engage in dietary behavior change. A “stages of change” approach to nutritional counselling should therefore follow the “Five As” approach successfully used in tobacco cessation,35 where members of the clinical care team ask about current behaviors, advise on healthful practices, assess readiness and motivation for change, assist in identifying individual goals, and arrange for referrals or other tools for those ready to use them. Such brief motivational interventions require less than five minutes of professional time, but typically must be offered on a repeated basis to be effective. This study demonstrates that most patients have the capacity to use tools such as the ASA-24 at home to create nutritional assessments, which could serve as the basis for nutrition-focused conversations with clinicians, emphasizing both areas of success, as well as goals for change.
Clinicians routinely invest in multiple discussions with patients to select among treatment options which may confer only marginally different survival benefits; nevertheless, such informed decision-making discussions are essential elements of good cancer care. If clinicians, survivors, and their families understand the growing evidence that, for many cancers, dietary behaviors offer advantages equally important as some clinical treatment decisions in improving good quality of life and disease-free survival, then nutrition education will become an essential and meaningful part of cancer survivor care.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NCI-NIH OBSSR Grant #1R21CA152789 (K. Smith, PI). Dr Coa and Dr Hannum were supported by NIH T32CA009314 (E. Platz, PI), and Ms Stoney was supported by NIH R25MD00679 (S. Marquez, PI).
References
- 1. Rowland JH, Kent EE, Forsythe LP, et al. Cancer survivorship research in Europe and the United States: where have we been, where are we going, and what can we learn from each other? Cancer. 2013;119:2094-2108. doi: 10.1002/cncr.28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: Cancer J Clin. 2016;66:271-289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3. American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 4. Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2013/. Accessed April 2016. [Google Scholar]
- 5. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25;1029-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Cancer Research Fund International. Continuous update project report: diet, nutrition, physical activity, and breast cancer survivors. 2014. http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf.
- 7. Joshu CE, Mondul AM, Menke A, et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res. 2011;4:544-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaflin HJ, Lee SB, Jeong BC, et al. Obesity and long-term survival after radical prostatectomy. J Urol. 2014;192:1100-1104. [DOI] [PubMed] [Google Scholar]
- 9. Pekemzi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198-2204. [DOI] [PubMed] [Google Scholar]
- 11. Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Dietary quality of cancer survivors and noncancer individuals: results from a national survey. Cancer. 2015;121:4212-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674-684. [PubMed] [Google Scholar]
- 14. Kenzik K, Pisu M, Fouad MN, Martin MY. Are long-term cancer survivors and physicians discussing health promotion and healthy behaviors? J Cancer Surviv. 2016;10:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker AM, Smith KC, Coa KI, et al. Clinical care providers’ perspectives on body size and weight management among long-term cancer survivors. Integr Cancer Ther. 2015;14:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coa K, Smith KC, Klassen AC, et al. Capitalizing on the ‘teachable’ moment to promote healthy dietary changes among cancer survivors: the perspectives of healthcare providers. Support Care Cancer. 2015;23:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith KC, Coa KI, Klassen AC. A qualitative study of dietary discussions as an emerging task for cancer clinicians. SAGE Open Med. 2016;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coa K, Smith KC, Klassen AC, Caulfield L, Thorpe R. Exploring important influences on the healthfulness of prostate cancer survivors’ diets. Qual Health Res. 2015;25:847-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith KC, Klassen AC, Coa K, Hannum S. The salience of cancer and the ‘survivor’ identity for people who have completed acute cancer treatment: a qualitative study. J Cancer Surviv. 2016;10:457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hannum SM, Smith KC, Coa K, Klassen A. Conceptualizations of the aging and aged self in ‘cancer survivorship’. J Psychosoc Oncol. 2016;34:477-492. [DOI] [PubMed] [Google Scholar]
- 21. Klassen AC, Smith KC, Black MM, Caulfield LE. Mixed method approaches to understanding cancer-related dietary risk reduction among public housing residents. J Urban Health. 2009;86:624-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jupp V. The SAGE Dictionary of Social Research Methods. Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- 23. Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012;112:1134-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willett W. Nutritional Epidemiology. 3rd ed. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 25. Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32:1054-1062. [DOI] [PubMed] [Google Scholar]
- 26. Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. SF-12v2™: How to Score Version 2 of the SF-12® Health Survey. Lincoln, RI: QualityMetric; 2002. [Google Scholar]
- 27. US Department of Health and Human Services. Healthy People 2020. Washington, DC: US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; http://www.healthypeople.gov/2020/topics-objectives/topic/physical-activity/objectives. Accessed September 2017. [Google Scholar]
- 28. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242-274. [DOI] [PubMed] [Google Scholar]
- 29. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, DC: Government Printing Office; 2010. [Google Scholar]
- 30. Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967. [Google Scholar]
- 32. An R, Liu J. Fast-food and full-service restaurant consumption in relation to daily energy and nutrient intakes among US adult cancer survivors, 2003-2012. Nutr Health. 2013;22:181-195. [DOI] [PubMed] [Google Scholar]
- 33. Low CA, Beckjjord E, Bovberg DH, et al. Correlates of positive health behaviors in cancer survivors: results from the 2010 LIVESTRONG Survey. J Psycholsoc Oncol. 2014;32:678-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazanec SR, Flocke SA, Daly BJ. Health behaviors in family members of patients completing cancer treatment. Oncol Nurs Forum. 2015;42:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larzelere MM, Williams DE. Promoting smoking cessation. Am Physician. 2012;85:591-598. [PubMed] [Google Scholar]