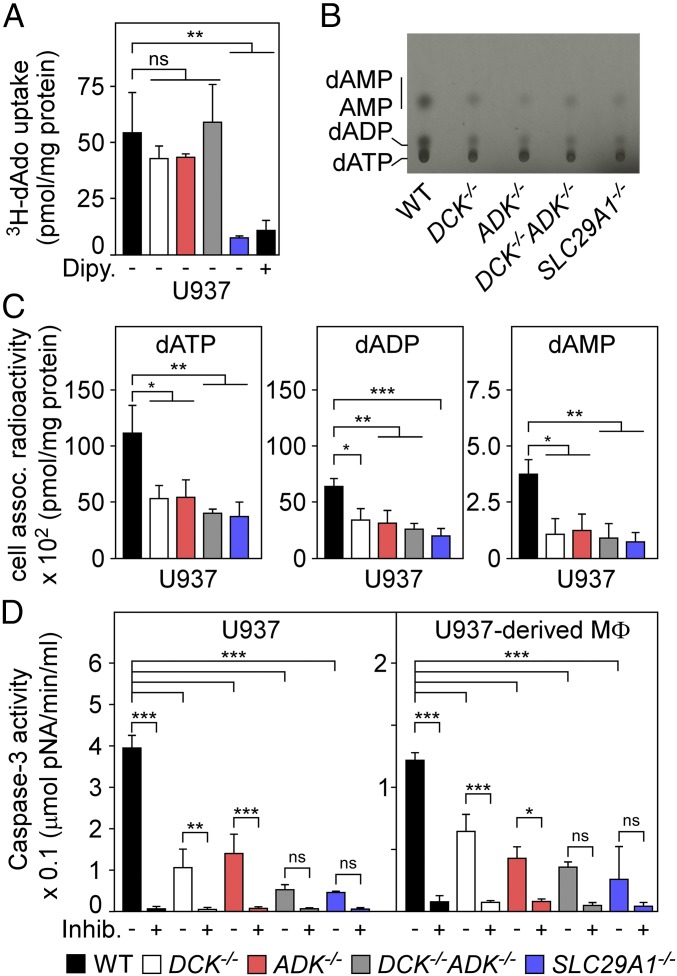
Fig. 4.
Mutations disrupting the purine salvage pathway of deoxyadenosine-intoxicated macrophages provide resistance to caspase-3–induced cell death. (A) Uptake of [3H]dAdo into wild-type U937 cells (WT, black) and their DCK−/− (white), ADK−/− (red), DCK−/− ADK−/− (gray), or SLC29A1−/− (blue) variants was quantified by LSC. As a control, dipyridamole (Dipy) (10 μM) inhibition of hENT1 blocked uptake of [3H]dAdo into WT U937 cells. (B) Analysis of [14C]dAdo-derived nucleotides from WT U937 cells and their DCK−/−, ADK−/−, DCK−/− ADK−/−, or SLC29A1−/− variants. An autoradiograph of the TLC plate is shown with migrational positions of dAMP, AMP, dADP, and dATP. (C) Nucleotides extracted from [14C]dAdo-treated cells were analyzed by TLC and LSC for the abundance of dATP, dADP, and dAMP. (D) WT U937 cells and U937-derived macrophages (MΦ) and their DCK−/−, ADK−/−, DCK−/− ADK−/−, or SLC29A1−/− variants were treated with dAdo and cell lysates were analyzed for caspase-3 activity using a colorimetric assay. As controls, lysates were treated with the caspase-3 inhibitor Ac-DEVD-CHO (+Inhib). All data are the mean (±SD) of three independent determinations. Statistically significant differences were analyzed with one-way ANOVA and Bonferroni’s multiple comparison test. ns, not significant (P > 0.05); *P ≤ 0.05; **P < 0.01; ***P < 0.001.