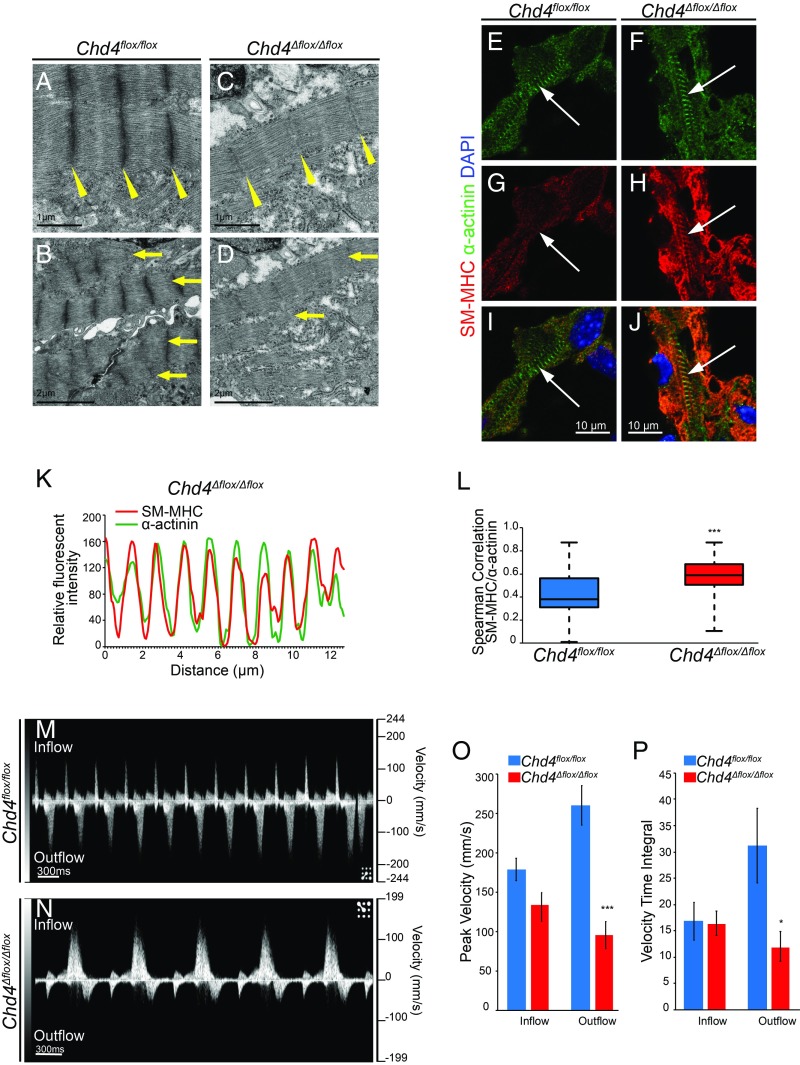
Fig. 4.
Misexpression of noncardiac myofibril paralogs in the absence of CHD4 leads to sarcomere malformation and altered cardiac function during development. (A–D) Transmission electron microscopy reveals weak, deficient Z-disc formation (yellow wedges) and decreased sarcomere formation and alignment (yellow arrows) in Chd4Δflox/Δflox hearts. (E–J) Costaining for α-actinin (E and F) and smooth muscle myosin heavy chain (SM-MHC) (G and H) demonstrates organization of SM-MHC into striated sarcomere structures and integration into the cardiac sarcomere in Chd4Δflox/Δflox cardiomyocytes compared with Chd4flox/flox controls (I and J). (K) Relative fluorescent signal of SM-MHC and α-actinin plotted against distance in E10.5 Chd4Δflox/Δflox cardiomyocytes reveals intercalation of SM-MHC into the nascent sarcomere. (L) Spearman correlation between SM-MHC/α-actinin signal in Chd4Δflox/Δflox cardiomyocytes is significant compared with Chd4flox/flox controls by Student’s t test, n = 27 vectors per genotype. (M and N) Noninvasive in utero embryonic echocardiography by pulsed-wave (PW) Doppler on E10.5 embryos shows Chd4Δflox/Δflox embryos have pronounced decreases in ventricular (outflow) velocity (N) compared with Chd4flox/flox controls (M). (O and P) Quantification of blood flow velocity from PW Doppler shows significant decrease in ventricular cardiac function in Chd4Δflox/Δflox hearts by peak velocity (O) and velocity time integral (VTI) (P) by Student’s t test, n = 5 embryos per genotype, SEM ±14.35, 18.12, 25.12, and 16.93 (O) and 3.57, 2.30, 7.11, and 2.85 (P). *P value < 0.05, ***P value < 0.001.